
The impact of the pandemic on the mental health of health care workers, including pharmacists and pharmacy staff, has been well documented.

The impact of the pandemic on the mental health of health care workers, including pharmacists and pharmacy staff, has been well documented.

Capecitabine (Xeloda) is indicated for adjuvant colon cancer, metastatic colorectal cancer, and metastatic breast cancer.
Treatment identifies patients with ROS1 fusion-positive non-small cell lung cancer or those with NTRK fusion-positive solid tumors for whom treatment with Rozlytrek may be appropriate.

In clinical trials, dabrafenib and trametinib showed meaningful efficacy in multiple BRAF-positive tumor types, including in patients with rare cancers who have no other treatment options available.

PAPs can significantly reduce OOP costs for patients.

Imlunestrant is currently under investigation as monotherapy or in combination with other anticancer therapies for patients with estrogen receptor-positive, locally advanced or metastatic breast cancer and endometrial cancer.

Even if the financial resources were available, there aren’t enough human resources in pharmacy to provide high-touch engagement to the millions of patients who suffer from common chronic diseases.

Part 1 of this 4-part series provides a general overview of the trends that are moving payers and pharmaceutical companies in this direction.
Tocilizumab (Actemra) is indicated for treatment of rheumatoid arthritis, giant cell arteritis, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.

The treatment landscape for relapsed/refractory MZL and follicular lymphoma (FL) is shifting from intravenous chemotherapy and monoclonal antibodies to oral oncolytic therapy or CD19-directed chimeric antigen receptor (CAR) T-cell therapy.

Pharmacists know that even the slightest delay in delivering critical medications can interrupt patient therapy schedules, negatively impact lives, and translate to millions of dollars in business losses

Social determinants of health (SDOH), which include social, economic, and physical conditions, have a major impact on people’s health, wellbeing, and quality of life.

Risankizumab-rzaa (Skyrizi) is the first and only specific interleukin-23 inhibitor approved for the treatment of adults with moderately to severely active Crohn disease.

The treatment gives patients with neovascular age-related macular degeneration a more affordable option, the companies say.
Utilization of the RAPID3 tool can be a beneficial way for pharmacists to complete disease activity assessments without physically seeing the patient in a clinic setting.
Selpercatinib (Retevmo, Loxo Oncology) is FDA-approved to treat 3 types of tumors—metastatic RET fusion-positive non-small cell lung cancer, advanced medullary thyroid cancer (MTC) or MTC that has spread, and advanced RET fusion-positive thyroid cancer.
Clinically meaningful results may be a huge step forward in the development of a new therapy for systemic lupus erythematosus.

Pembrolizumab is an anti-PD-1 therapy that works by increasing the ability of the immune system to detect and fight tumor cells.

Although the COVID-19 pandemic saw the rapid hemorrhaging of women from careers in science and medicine, research shows the toxicity experienced by women may be structural in nature.

Current development efforts in IVIG are focused on new routes of administration for immunoglobulin that can overcome the limitations of intramuscular administration.
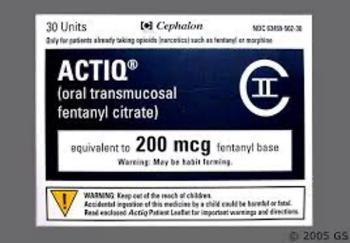
Fentanyl citrate (Actiq) is an opioid agonist indicated for the management of breakthrough pain in cancer patients.
Many health care systems are turning to biosimilars to provide clinical outcomes that are similar to those provided by reference products, but at a reduced cost.

As best practices are established throughout the specialty pharmacy’s entire network, better patient outcomes become possible.

Tina Saleh, PharmD, PGY1 specialty pharmacy resident at the University of Illinois Hospital and Health Science System, discusses how oral anticancer agents differ from infusions and how pharmacists can improve tolerability for patients.

Shields Health Solutions poster presentations highlight a comprehensive approach to specialty patient care and successful results from an expanded offering of instructional programs.

Baricitinib (Olumiant; Eli Lilly and Company) is the first systemic treatment to be approved by the FDA for severe alopecia areata, which affects an estimated 300,000 patients in the United States.
These results from 3 separate long-term pooled analyses of adult patients with ulcerative colitis (UC) and CD treated with ustekinumab (Stelara) were presented at Digestive Disease Week meeting in San Diego, California.
In an analysis of data from 2 patient cohorts included in the phase 2 BYLieve study, investigators found that the data may indicate an increased dependence on the mutant PI3K-α.
JZP458, a recombinant Erwinia-derived ASNase from a Pseudomonas fluorescens expression platform, was previously approved by the FDA for patients with ALL/LBL who had developed hypersensitivity to Escherichia coli (E. coli)–derived ASNase.
Investigators reported that individuals who completed 10 cycles of lurbinectedin and doxorubicin who then switched to lurbinectedin as a monotherapy tended to have maintained or improved tumor response.