The approval is based on data from Study 5310, evaluating the pharmacokinetics, safety, and efficacy of Biktarvy (Gilead Sciences Inc) in pregnant individuals.

The approval is based on data from Study 5310, evaluating the pharmacokinetics, safety, and efficacy of Biktarvy (Gilead Sciences Inc) in pregnant individuals.

With migraine as a leading disability for those under the age of 50, treatment options for migraine should be personalized to the patient and their needs.
Frexalimab demonstrated a favorable tolerability profile after approximately 1 year of treatment for individuals with relapsing disease.
Ofatumumab (Kesimpta; Novartis) demonstrated a sustained efficacy as a first-line, continuous treatment for patients recently diagnosed and treatment-naïve with relapsing multiple sclerosis.
The trial will compared the efficacy, safety, pharmacokinetics, and immunogenicity between SB27 and pembrolizumab for metastatic non-squamous non-small cell lung cancer.

Ocrelizumab (Ocrevus; Roche) is being investigated as a twice yearly 10-minute subcutaneous injection compared to the intravenous infusion formulation.

Adrijana Kekic, PharmD, a pharmacogenomics clinical specialist with Mayo Clinic in Phoenix, Arizona, discussed current guidelines for pharmacogenomics.

Eli Lilly and Company will submit results of the study to support tirzepatide (Zepbound) for treatment of obstructive sleep apnea later this year.
Ustekinumab (Stelara; Janssen Immunology) is a human monoclonal antibody that treats immune-mediated diseases such as psoriasis and psoriatic arthritis.
If approved, the test could provide more timely and accurate diagnosis, hopefully mitigating the impact of Alzheimer disease (AD) on individuals and the community.

Sa’ed Al-Olimat, PharmD, co-founder of the Psychedelic Pharmacists Association, discussed the future of psychedelic medicines and the unique ethical challenges they present.
There was no difference observed between adalimumab (Humira; AbbVie) and adalimumab-aacf (Idacio; Fresenius Kabi) for those who switched to the biosimilar.

However, psychedelic medicine has a variety of barriers, including historical perception, access challenges, legal obstacles, and ethical questions.

In a review, investigators focused on ranibizumab (Lucentis; Genentech), aflibercept (Eylea; Regeneron), and bevacizumab (Avastin; Genentech) as growth opportunities in the biosimilar space.

The treatment is indicated for adolescents at least 12 years of age who weigh at least 25 kg without a previous treatment history of antiretroviral therapies.
The approval expands the prior indication of idecabtagene vicleucel (Abecma; Bristol Myers Squibb), which will make the drug available to patients in earlier lines.

Bimekizumab-bkzx (Bimzelx; Union Chimique Belge) is a humanized monoclonal immunoglobulin G1 antibody that inhibits interleukin (IL)-17A and IL-17F.

The results indicate the need for targeted policies so that biosimilar competition can increase cost savings and affordability for patients with commercial insurance.

Advanced analytics will continue to make health care more accessible and empower pharmacists with the ability to personalize treatments.

By implementing easier systems of education for patients, the consequences of polypharmacy could potentially decrease.
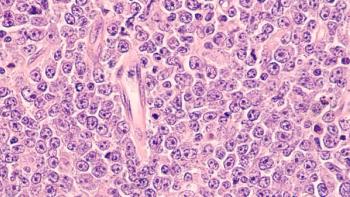
The mutation impacts BAF proteins and can lead to progression of follicular lymphoma, according to the investigators.
Investigators found that mRNA technology for the delivery of antibody therapeutics were used to target tau in Alzheimer disease and can be applied to other tau targets.
The new indication includes pediatric patients aged 6 years and older who weigh at least 25 kg and have compensated liver disease.

Vadadustat (Vafseo; Akebia Therapeutics Inc) is indicated for individuals with chronic kidney disease who have been receiving dialysis for at least 3 months.

Just as the pharmacy field is expanding its horizons, CE programs are offering new topics and learning strategies.

Psychedelic medicines such as psilocybin, MDMA, ketamine, and LSD have been shown to have a significant impact on conditions such as major depressive disorder, generalized anxiety disorder, and post-traumatic stress disorder in clinical trials.

An FDA executive discusses the Surgeon General’s recommendations for promoting mental health and wellbeing for employees in the workplace.

Sa'ed Al-Olimat, PharmD, also discussed the potentially groundbreaking shift for MDMA expected later in 2024.

The FDA recommends the dosage of givinostat (Duvyzat; Italfarmaco, ITF Therapeutics) should be based on the individual’s body weight and administered twice daily with food.

The BLA includes all indications for Xolair, including asthma, chronic rhinosinusitis with nasal polyps, immunoglobulin E-mediated food allergy, and chronic spontaneous urticaria.