A 1-time infusion of valoctocogene roxaparvovec had demonstrated sustained bleed control and factor VIII expression in patients with hemophilia A.

A 1-time infusion of valoctocogene roxaparvovec had demonstrated sustained bleed control and factor VIII expression in patients with hemophilia A.
The default-mode network method, which was analyzed using functional magnetic resonance imaging scans, was approximately 80% accurate when predicting dementia up to 9 years prior to a diagnosis.
Individuals with very low- to intermediate-risk myelodysplastic syndromes treated with luspatercept (Reblozyl; Bristol Myers Squibb) achieved improvements in hemoglobulin levels.
Elafibranor (Iqirvo; GENFIT) is a first-in-class, once daily oral peroxisome proliferator-activated receptor for the treatment of primary biliary cholangitis.
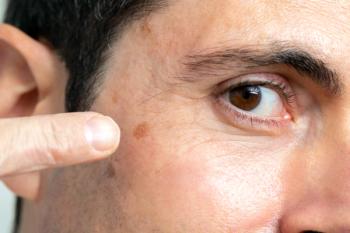
The new approval increases the previous dosing for surface area treatment from 25 cm2 to up to 100 cm2.

The authors noted that these networks have a significant role in intellectual ability, physical coordination, working memory, emotional processing, and overall mental health.

Imetelstat (Rytelo; Geron Corp) is a first-in-class telomerase inhibitor.
According to investigators, the primary and key secondary end points were met during the 52-week placebo-controlled period of the trial.

With its ability to decode genetic variations influencing drug response, pharmacogenomics holds immense promise in reshaping health care delivery.

Mirikizumab met both co-primary end points and all secondary end points at week 52, according to results presented at Digestive Disease Week in Washington DC.
For patients with a blood eosinophil count of 150 cells/µL or greater, tezepelumab had a significant reduction of approximately 37% in the rate of moderate or severe exacerbations.

The panelists expressed concerns about clinical trial data and concluded that the benefits do not outweigh the risks, but the FDA does not have to follow their recommendation.

In addition to racial inequities in access to care, socioeconomic challenges are very common for the specialty medications necessary in MS.

Providers, the community, health systems, and health policies can all play a part in addressing various challenges in care for obesity.

Understanding patients’ specific experiences and challenges is crucial to helping them improve medication adherence.

This is the largest dataset of pregnancy outcomes for an anti-CD20 therapy in MS, enabling a more comprehensive understanding of ocrelizumab’s safety.

In addition to medication management, pharmacists are educating patients and helping them navigate the complex medical system.

Although this data seems to overwhelmingly suggest that cannabis use has negative impacts for individuals with multiple sclerosis (MS), listening to patients’ reasoning for using it is crucial.
Subcutaneous ocrelizumab can be delivered in approximately 10 minutes and deliver comparable clinical benefit and safety to the IV formulation.
Lisocabtagene maraleucel (Breyanzi; Bristol Myers Squibb) is a CD19-directed CAR T-cell therapy that is delivered as a one-time infusion.

Although resources were scarce for teams at smaller private practices, the pharmacists all agreed that patient care is always the priority.
There has been a general shift toward earlier treatment and less tolerance for clinically silent disease activity.

Although questions remain about ideal treatment strategies, consistent data have shown that patients should be treated early.
Eculizumab-aeeb (Bkemv; Amgen) is approved for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.

Added data from the SELECT trial helps to increase confidence in safety and efficacy of semaglutide, a glucagon-like peptide 1 medication.

Australia, Québec, and Sweden have defined processes for reimbursements by health technology assessments, but the United States has a low reliance rate.

Building a network, finding your passion, and taking steps to transition into an industry professional can help lead PharmD graduates to success.

The GLP-1 market is expected to exceed $100 billion by 2023, according to J.P. Morgan Research, with total users in the United States projected to reach 30 million.

Aflibercept-jbvf (Yesafili; Biocon Biologics) and aflibercept-yszy (Opuviz; Biogen, Samsung Bioepis) are the first interchangeable biosimilars to aflibercept (Eylea; Regeneron).
Belimumab (Benlysta; GSK) is a B-lymphocyte stimulator-specific inhibiting monoclonal antibody.