
Top news of the day from across the health care industry.

Top news of the day from across the health care industry.

Eric Dallara, RPh, pharmacist for the New England Cancer Specialists explains the current challenges in the industry, especially regarding payment plans and payment reform during the National Community Oncology Dispensing Association, Inc. in Denver, Colorado.

A recent study showed that flavonoid-rich cocoa improved fatigue by 45% in patients with relapsing and remitting multiple sclerosis.

Top news of the day from across the health care industry.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

Study provides insight into how prostate cancer transitions to an aggressive, treatment-resistant subtype following anti-androgen therapy.
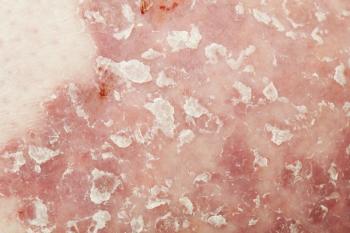
A head-to-head trial evaluated quality of life improvements in patients with moderate-to-severe psoriasis treated with secukinumab (Cosentyx) or ustekinumab (Stelara).

Top news of the week from Specialty Pharmacy Times.
Vaginal inserts containing tenofovir alafenamide fumarate in combination with elvitegravir were shown to be highly effective in preventing simian/HIV infection in a macaque model.

Alvotech’s AVT02 is being developed as a biosimilar to the adalimumab (Humira, AbbVie) high-concentration product.

Phase 3 trial evaluated ustekinumab as a maintenance treatment in adult patients with moderate-to-severe ulcerative colitis.

Top news of the day from across the health care industry.
Study provides new evidence on the pharmaceutical industry-led medicines access program.

The latest sBLA is based on a phase 3 study evaluating the daratumumab combination regimen for newly-diagnosed patients with multiple myeloma who are ineligible for transplantation.
Study suggests that a fasting diet can reduce inflammation and increase intestinal stem cells in patients with inflammatory bowel disease.

Top news of the day from across the health care industry.
Women with multiple sclerosis who breastfed exclusively for at least 2 months had a 40% lower rate of relapse compared with women who did not breastfeed.

Trastuzumab is indicated for the treatment of HER2 overexpressing breast cancer and HER2 overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma.
PARP inhibitors used alongside immunotherapy may help stimulate the immune response to cancer cells by attracting immune cells to the tumor.

Top news of the day from across the health care industry.

The study was the first to compare patients with hepatitis C treated with direct-acting antiviral drugs and those untreated for long-term risks.

Stem cell transplantation found to provide greater protection than disease-modifying therapy against progression of relapsing-remitting multiple sclerosis.

Ali McBride, PharmD, MS, BCOP, FASHP, FAzPA discusses the emerging influence of NCODA chemotherapy education and standardization process for healthcare providers, healthcare systems, and patients.
The ever-growing price tag for new specialty drugs is testing the limits of the health care system to afford these revolutionary therapies.

With this approval, dupilumab (Dupixent) offers a biologic therapy option for patients 12 to 17 years of age who have moderate-to-severe atopic dermatitis.

Cheryl Allen, BSPharm, MBA, Vice President of Industry Relations at Diplomat Pharmacy, talks about new therapeutic developments going on within the prostate cancer space.

Top news of the day from across the health care industry.
M restricta was elevated in patients with Crohn disease carrying a genetic variation known as the IBD CARD9 risk allele.

Because pregnancy is one of the few times individuals are in contact with the health system and covered by health insurance, there is a cost-effective opportunity to screen for hepatitis C virus.

The FDA recently published an updated draft guidance on the naming of biologics, biosimilars, and interchangeable biosimilars.