
The cost of oncology treatment is high and rising in the United States.
Rami Komrokji, MD, clinical director of malignant hematology and department lead clinical investigator at the H. Lee Moffitt Cancer Center and Research Institute speaks about why data are limited in myelofibrosis.

For patients with relapsed/refractory multiple myeloma (RRMM), teclistamab was found to have a manageable safety profile, according to data presented at the virtual American Society of Hematology conference.
The data presented at ASH 2020 build on results previously observed in the pivotal HAVEN clinical trial among adults, adolescents, and children with hemophilia A, with and without factor VIII inhibitors.

Supreme Court decision establishes that states do have the right to regulate pharmacy benefit managers in managed care organizations.
Rami Komrokji, MD, clinical director of malignant hematology and department lead clinical investigator at the H. Lee Moffitt Cancer Center and Research Institute speaks about factors in treatment decisions for patients with myelofibrosis.

Data from a preliminary phase 1b/2 study demonstrated a single low-dose infusion of ciltacabtagene autoleucel resulted in early, deep, and durable responses in heavily pretreated patients with multiple myeloma.

MEDI2228, an ADC that targets the extracellular domain of human BCMA, demonstrated clinical efficacy at all dose levels in treating patients with relapsed/refractory multiple myeloma.
Trials confirm patients with chronic lymphocytic leukemia treated with venetoclax-based regimens achieve higher rates of undetectable minimal residual disease, which may be associated with a lower risk of future disease progression or death.
An icatibant injection has been released in the United States as an alternative treatment for acute attacks of hereditary angioedema in adults over the age of 18 years.
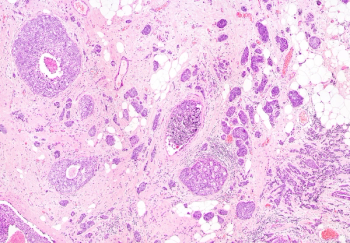
Although postoperative radiation therapy affected the risk of local recurrence of breast cancer, it did not significantly impact certain other clinical outcomes, including distant metastasis and recurrence in the opposite breast.
The higher risk of delivery and fetal complications suggests that physicians should more closely monitor pregnant breast cancer survivors.

Session at at the San Antonio Breast Cancer Symposium featured several abstract presentations on disparities in health care access in breast cancer, including research on the necessity of avoiding a one-size-fits-all approach to medicine.
Younger and Black patients are more likely to experience breast cancer symptom under-recognition.

Overdiagnosis of breast cancer can lead to unnecessarily aggressive treatments and mastectomies.

If current drug pricing trends continue, cost-related non-adherence to therapy will result in the premature deaths of 112,000 Medicare beneficiaries per year.

Grief can affect an organization as well as an individual; however, there are management strategies that can be used for addressing these issues.
There were significant modifications in breast cancer treatment due to the coronavirus disease 2019 pandemic, such as high rates of NET chemotherapy, genomic assay testing on core biopsies, and delays in planned surgeries.
Tesetaxel is a novel, oral taxane with several unique properties being investigated for use in patients with HER2-negative, HR-positive metastatic breast cancer.
Acquired hemophilia A is a rare bleeding disorder caused by autoantibodies that inhibit coagulation factor VIII (FVIII), and the disorder is understudied given its rarity and the lack of randomized prospective trials to guide therapy.

ET and CT are used as standard maintenance therapy for HR-positive and HER-negative MBC in clinical practice, and there was no prospective study data on which is better, according to the study authors.
BIVV001 is currently being studied as a recombinant factor VIII treatment for hemophilia A.
A single infusion of damoctocog alfa pegol resulted in 25% higher area under the curve and 20% lower clearance in patients with severe hemophilia A.
Belantamab mafodotin is a B-cell maturation antigen-targeting antibody-drug conjugate being investigated in the treatment of multiple myeloma.

Intravenous immunoglobulin administered to hypoxic non-ventilated COVID-19 patients with an A-a gradient of more than 200 mg Hg significantly decreases the rates of progression to mechanical ventilation.
The proportion of patients with hemophilia A receiving Hemlibra who experienced zero treated bleeds increased with each consecutive 24-week period.
Eligible patients in the APOLLO study had RRMM and received more than or equal to 1 prior line of therapy including lenalidomide and a proteasome inhibitor (PI) and had responded to prior treatment and progressed on or after their last regimen.
AMG 701 is a bi-specific T-cell engager molecule being investigated for relapsed or refractory heavily pre-treated multiple myeloma.
Melflufen plus dexamethasone as a triple regimen with bortezomib and daratumumab in patients with heavily pretreated relapsed or refractory multiple myeloma with poor prognostic factors was well-tolerated.
Talquetamab is a bispecific antibody that binds to GPRC5D and CD3 to induce T cell-mediated killing of GPRC5D-expressing multiple myeloma cells.