
Omidubicel is a first-in-class, advanced nicotinamide-enabled stem cell therapy candidate being evaluated as the first potential allogeneic advanced cell therapy donor source for individuals with blood cancers in need of a transplant.

Omidubicel is a first-in-class, advanced nicotinamide-enabled stem cell therapy candidate being evaluated as the first potential allogeneic advanced cell therapy donor source for individuals with blood cancers in need of a transplant.

FDA approves 15 mg upadacitinib (Rinvoq; AbbVie) for the treatment of adult patients with active ankylosing spondylitis who previously showed an inadequate response or intolerance to 1 or more tumor necrosis factor inhibitors.
The advanced cell therapy is under development as an allogeneic hematopoietic stem cell transplant for patients with hematologic malignancies.

Stay tuned at PharmacyTimes.com for exclusive session coverage and interviews with experts.
Acute graft-versus-host disease (aGVHD) is the most frequently encountered, life- threatening complication following an allogeneic hematopoietic stem cell transplant (aHSCT).

Ravulizumab-cwvz (Ultomiris) is the first and only long-acting C5 complement inhibitor for the treatment of generalized myasthenia gravis to gain FDA approval.
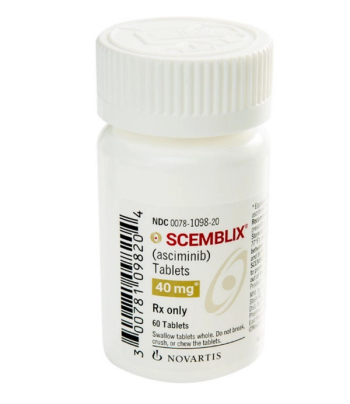
Scemblix is indicated for Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase, previously treated with 2 or more tyrosine kinase inhibitors.

The data show that the 10-year survival rate of patients with breast cancer who survived Hurricane Katrina was much lower than those who did not face a major weather event.
Two-thirds of women diagnosed with endometrial cancer have localized disease and a 5-year relative survival of 95%.
Up to 50% of all patients with breast cancer have tumors with a HER2 immunohistochemistry level not currently eligible for HER2-targeted therapy.
STRIDE regimen of a single priming dose added to Imfinzi is the first dual immune checkpoint blockage regimen to improve overall survival in a phase 3 trial in this setting.
An expert panel engaged the audience in a live virtual symposium and case discussion on immune checkpoint inhibitors (ICIs) in the first-line treatment of non−small cell lung cancer (NSCLC) at the 2021 ASHP Midyear Clinical Meeting.
Because of the likelihood that patients with melanoma will develop adverse effects on therapy, it is crucial for pharmacists to proactively educate patients and provide them with tools to help mitigate and manage these issues.

As pharmacy technicians continue to take on more responsibilities, actions must be taken to alleviate the burnout so many are facing.

Attendees of this live virtual program learned the latest and greatest in a presentation from 2 expert oncology pharmacists.

Psychedelic medicines like psilocybin work to re-wire the brain, potentially addressing the root cause of eating disorders rather than the symptoms.

DuoBody, for treatment of relapsed/refractory large B-cell lymphoma, demonstrates an overall response rate of 63.1%.
In a live virtual symposium at the 2021 ASHP Midyear Clinical Meeting, 2 experts teamed up to highlight the place in therapy and management of oral oncolytics in follicular and marginal zone lymphoma.

Elaine Jaffe, MD, National Institutes of Health (NIH) distinguished investigator at the National Cancer Institute at NIH, offers guidance for female medical researchers who are faced with a declining number of female colleagues in the workforce.

Kelan Thomas, PharmD, MS, discussed potential adverse effects and drug-drug interactions that pharmacists should be aware of with psychedelic medicines.

The updated efficacy analyses showed that tislelizumab in combination with chemotherapy continued to demonstrate a clinically significant progression-free survival benefit for individuals with recurrent or metastatic nasopharyngeal cancer.

The analyses are shared at the American Association for Cancer Research Annual Meeting 2022 in New Orleans, Louisiana.
The agent is the first oral therapy for this indication.

Investigational CAR T-cell therapy is currently being evaluated in an ongoing dose escalation phase 1 trial to analyze its safety and tolerability in treating non-Hodgkin lymphoma.

Elaine Jaffe, MD, National Institutes of Health (NIH) distinguished investigator at the National Cancer Institute at NIH, discusses where she sees the classification of lymphoma progressing in the coming years.
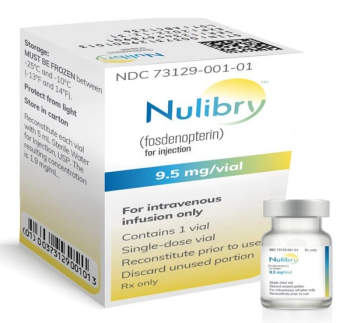
Fosdenopterin (Nulibry) is indicated to reduce the risk of mortality in patients with molybdenum cofactor deficiency Type A.
Sonia Oskouei, PharmD, vice president of biosimilars at Cardinal Health, discusses what biosimilars in the pipeline are expected to launch this year.

Analyses are shared at the American Association for Cancer Research Annual Meeting 2022 in New Orleans, Louisiana.

Sonia Oskouei, PharmD, vice president of biosimilars at Cardinal Health, on the results of Cardinal Health’s assessment of the current biosimilars market published in the 2022 Biosimilars Report.
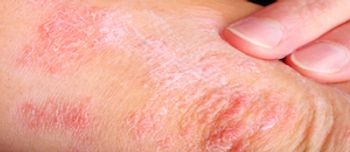
The phase 3, multicenter, randomized, double-blinded, comparative clinical study was evaluating the efficacy and safety of ABP 654 compared with ustekinumab in adult patients with moderate to severe plaque psoriasis.