
The recent World Health Innovation Forum investigated the use of artificial intelligence in the medical field. Here are some takeaways.

The recent World Health Innovation Forum investigated the use of artificial intelligence in the medical field. Here are some takeaways.

Stuck at work for a 12 hour shift? Here are easy ways to fit in more activity during your shift.
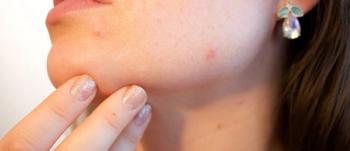
Walgreens has introduced an online skincare analysis tool called SkinID to evaluate each customer’s skincare needs, and build a personalized, 3-step daily routine that will help banish breakouts.

Where does a pharmacist start after being released into the open job market?

Obstetricians and general practitioners most often field questions from women who have asthma, as this condition is more common than most other chronic conditions in young people.

The Walgreens Flu Index shows which populations are experiencing the highest incidences of influenza each week, based on Index methodology.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

Upgrades to the insulin pen to improve disease management and prevent hypoglycemia due to overdoses are on the horizon.

Procter & Gamble (P&G) announced last month that it has agreed to acquire Merck KGaA’s consumer health unit for 3.4 billion euros or about $4.2 billion.

Make sure that all individuals involved are exposed to the same process and treated equally, even though the infractions may differ.

The recent Escherichia coli (E.coli) outbreak linked to romaine lettuce is extremely virulent and has affected 22 states across the United States.

Here is the lowdown on this nutrition plan, which is all the rage.

In this last installment of the series, our expert pharmacists weigh in on a variety of issues

As the big day approaches, students may be experiencing many emotions. Here is how to get through the ceremony.

Those who have been putting off making vacation plans or taking personal days should think again.

Pharmacists in all practice settings have opportunities to assist patients who use or seek to use contraception.

Pharmacists can help identify pharmaceuticals that could trigger symptoms in those with the autoimmune condition.

Medication is not cheap, but we can help ensure that cost is not a barrier to adherence. Here are some tips.

Fewer than 1 in 10 pharmacists talked to most patients about asthma action plans routinely.

A recent report about a person who stole a neighbor's medications in a suicide attempt provides food for thought.

New study results show that this type of blood pressure measurement is a better predictor of mortality than in-clinic assessments.

This will both enhance a pharmacist's knowledge and improve his or her writing skills.

One Drop is a company that has a great holistic service using CDE to help patients get their diabetes better controlled, and recent data support their mission.

Pharmacists, it turns out, can impact their own outcomes by the kinds of people they spend time with most often.

Milk thistle, which contains an active component called silymarin, has been used for centuries for disorders of the liver, and bile duct.

The FDA has issued a drug safety communication that the epilepsy and bipolar medication lamotrigine (Lamictal) can cause a rare, serious adverse effect known as hemophagocytic lymphohistiocytosis (HLH).

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

Working in a pharmacy is busy and not always conducive to eating well. Here are some tips for staying on the right track.

There are so many protein powders on the market. How do you know which is right for you or your patients?

Solanezumab did not significantly affect cognitive decline compared with the placebo in subjects with mild Alzheimer's, but there are still takeaways.