
Laura Davis Taylor and Ed King, who are consultants on retail sales strategy, discuss some key take away points from their presentation at the National Association of Chain Drug Stores (NACDS) Total Store Expo,

Laura Davis Taylor and Ed King, who are consultants on retail sales strategy, discuss some key take away points from their presentation at the National Association of Chain Drug Stores (NACDS) Total Store Expo,

Advice for patients who are considering menstrual cups.

Patient outcomes are often the result of what patients do when they are away from their providers.

Can you solve the pharmaceutical mystery? Each week, a new case study is presented.
Serious vascular events in patients with diabetes can be prevented with aspirin use, according to results of the ASCEND (A Study of Cardiovascular Events iN Diabetes) trial, which were presented at the ESC Congress in Munich, Germany.

The benefits of taking aspirin to prevent a second or subsequent cardiovascular event have been well established in previous studies but the effectiveness of taking aspirin to prevent a first cardiovascular event has been unclear, despite 30 years of randomized clinical trials.

Medical cannabis has been a hot topic of discussion in the state of Colorado.

The adoption of digital health options is growing rapidly, with more than 318,000 health related apps available in the app store on most smart phones, and almost 200 more being added each day.

Speaker at NACDS Total Store Expo explains how Facebook and Instagram can boost retail pharmacy performance.

Watch as our Executive Vice President for Pharmacy Advocacy discusses pharmacy roles in immunizations, and record his "word of the day" for a chance to win $1000 gift card!

The data demonstrated that the lotion resulted in statistically significant reductions in both inflammatory and noninflammatory lesions compared to vehicle.


This week's featured pharmacist is Lisa Lohr, Pharm D, BCPS, BCOP, a Specialty Medication Therapy Management pharmacist specializing in oncology care.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

Officials with the FDA announced Thursday approval of lanadelumab (Takhzyro) for treatment of hereditary angioedema (HAE).
A new HIV therapy demonstrated its efficacy in reducing the virus and boosting immunity in drug-resistant patients, according to a recent published study.
Buprenorphine with naloxone (Suboxone), an established treatment for opioid use disorder, has been increasingly used in states that chose to expand Medicaid eligibility under the Affordable Care Act, according to the results of a recent study.
Officials with the FDA have approved a topical eye drop containing cenegermin, the first drug indicated for the treatment of a rare disease affecting the cornea.

The latest recommendation from the United States Preventive Services Task Force said cervical cancer screening depends on a woman’s age and other factors.

An evolving pharmacy practice started with the the owners' vision, and worked backwards to become a reality.

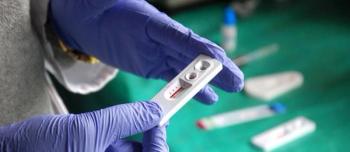
According to a recent study, older adults and Hispanics are less likely to receive HIV testing, indicating an urgent need for improved interventions.

Officials with the FDA have expanded the indication of pembrolizumab (Keytruda, Merck) for patients with metastatic, non-squamous non-small cell lung cancer (NSqNSCLC).

Officials with the FDA approved have approved stiripentol (Diacomit) for the treatment of seizures associated with Dravet syndrome, a rare form of epilepsy.
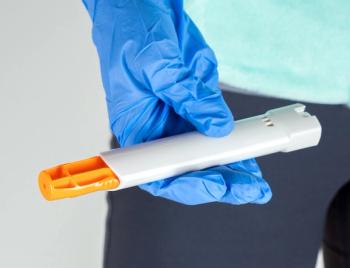
The FDA said that it has extended expiration dates on some EpiPen and generic versions, all manufactured by Pfizer, by 4 months.

Pharmacy students should take the pressure off themselves and think about their next steps, not the rest of their careers.

The prescribing limits are designed to help prevent patients from receiving unnecessary opioid pills after surgery.
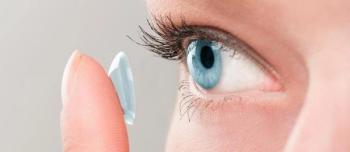
The CDC is Partnering with the American Optometric Association to bring inform patients about proper lens care, during Contact Lens Awareness Week.

In this clip, Kimberly Wu, a PharmD candidate at the Rutgers University Ernest Mario School of Pharmacy, discusses why it is important for pharmacy students to be proactive in their education, activities, and work.

Officials with the FDA have given approval for marketing Brainsway’s Deep Transcranial Magnetic Stimulation System for treatment of obsessive compulsive disorder.