
Why was a patient prescribed something normally used for stomach problems to help her produce breastmilk?

Why was a patient prescribed something normally used for stomach problems to help her produce breastmilk?

Program establishes criteria to specify potentially inappropriate medications to avoid prescribing to older adults or should be used with caution when combined with other prescriptions.

Today, we’re celebrating the work of Nidhin Mohan, RPh, from New Island Pharmac y in Deer Park, New York.

A new study showed that most women living with HIV that were interviewed preferred long-acting injectable adherence to antiretroviral therapy over daily pills.
Top news of the week from Pharmacy Times® surrounding the novel coronavirus.

Some pertinent information pharmacists should know about oral semaglutide

According to the results of a nationwide survey conducted among clinical pharmacists, too many working hours is the major factor causing burnout, followed by inadequate management.

With additional state action to remove testing restrictions, pharmacists will be able to continue their work to serve on the frontlines of health care during this pandemic.

Two case studies have highlighted the use of tele-diabetes to manage new-onset type 1 diabetes (T1D) cases in an adult and an infant during the coronavirus disease 2019 (COVID-19) pandemic.

Pharmacy managers must be especially adept at negotiating with various stakeholders, and pass on their negotiating skills to other pharmacy employees.
A new study indicates that treatment with hydroxychloroquine increases overall mortality in patients hospitalized with COVID-19.

Implementation of adherence programs can be effective in improving outcomes for patients and reduce costs both at the patient and systemic level.
Patients with type 2 diabetes (T2D) do not secrete enough insulin and secrete too much glucagon, contributing to poor blood glucose because the glucagon-secreting α-cells have become resistant to insulin.

Today, we’re celebrating Katherine Hicks, RPh, for her work at the Community Medical Clinic of Aiken County (CMCAC) in South Carolina.
Statin therapy is known to reduce the risk of cardiovascular events, and guidelines recommend them in all patients with diabetes.
Obesity increases the risk of developing type 2 diabetes by at least 6 times, regardless of genetic predisposition, according to a recent study.

The FDA has approved the first diagnostic test with a home collection option for the coronavirus disease 2019.
The new guidance authorizes licensed pharmacists to order and administer COVID-19 tests, including serology tests, that are approved by the FDA.

Today, we’re celebrating Hany Youssef, RPh, owner of LIFETIME Pharmacy in Allen, Texas.

Pharmacy Times® invites you to join us and experts in pharmacy to gain insight into the value pharmacists provide in treating patients throughout the pandemic.

More than 53% of survey respondents reported having a diversion event within the last year, in addition to 37% who were aware of at least 1 colleague who has diverted controlled substances.

An overview of the most recent legal developments related to COVID-19 that are relevant to pharmacies.
This cohort included 307 patients with schizophrenia and 364 healthy controls who were 45 years old and younger.

Widely used medications for patients with HIV/AIDS may be potential treatments for the coronavirus disease 2019 should the drugs demonstrate safety and efficacy in clinical trials.

Researchers from the Mayo Clinic have found that inappropriate use of high-potency opioids has contributed to the opioid epidemic in the United States.

Why is this patient on oxybutynin if he does not have any problems with his bladder?

Individuals with type 2 diabetes (T2D) who also experience coronary artery disease (CAD) may need to be treated more aggressively than those with coronary artery disease who do not have diabetes.

Adam Martin, PharmD, founder of the Fit Pharmacist, discusses tips for staying hopeful and motivated.
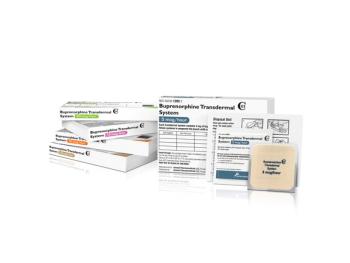
The FDA granted Amneal Pharmaceuticals for a generic version of buprenorphine (Butrans) transdermal system as well as a Competitive Generic Therapy designation.

Many health care companies are taking the opportunity to deliver testing kits to local pharmacies to properly equip pharmacists with the right resources during the pandemic.