
Data shed light on brentuximab vedotin plus nivolumab, bendamustine, or gemcitabine.

Data shed light on brentuximab vedotin plus nivolumab, bendamustine, or gemcitabine.

Currently, the safety and efficacy of linvoseltamab in adult patients with relapsed or refractory multiple myeloma is being compared to elotuzumab, pomalidomide, and dexamethasone in a phase 3 clinical trial.

Precision oncology represents an evolution in therapeutic practice.

Currently, ocifisertib is being evaluated in a phase 1b and 2 trial to confirm the safety and tolerability profiles in patients with acute myeloid leukemia.

Teclistamab was previously approved in October 2022 for the treatment of adult patients with relapsed or refractory multiple myeloma who received at least 4 prior therapies.
BAT1706 was clinically equivalent to the reference bevacizumab for efficacy, safety, pharmacokinetics, and immunogenicity.
Smoking 20 cigarettes per day significantly increased risk of death caused by certain types of melanomas.

These combination therapies have demonstrated longer PFS with a tolerable AE profile.
Datopotamab deruxtecan is an investigational TROP2 directed antibody drug conjugate that showed positive survival impact compared to chemotherapy.
If vorasidenib receives full approval, it would be a first-in-class target therapy to treat individuals with IDH-mutant gliomas.

Researchers from Gilead remain confident for positive results as they continue the phase 3 EVOKE-03 study in 1L metastatic PD-L1-high NSCLC.
Certain patients may present asymptomatically, affecting treatment options.
Osimertinib was previously approved as a monotherapy, the first-in-line global standard of care, for non–small cell lung cancer indications.

The pharmacist’s role may vary across different practices and institutions.
This marks the first and only time that an individualized T cell therapy has been approved by the FDA for a solid tumor cancer.
Tigilanol tiglate is a novel, small molecule drug for the intratumoral treatment of solid tumors, inducing tumor cell death and stimulating immune response.
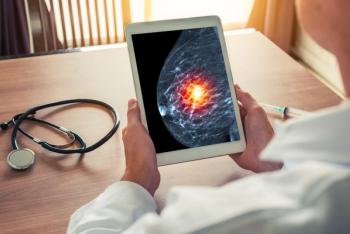
Tucatinib significantly improved median PFS in the overall population and those with brain metastases.

The complexity of the disease and number of treatment options can necessitate a personalized approach.

The investigators note that patients with newly diagnosed multiple melanoma who receive quadruplet treatment have unmet needs that must be further examined in future research.
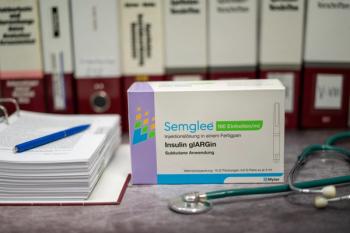
Since the first FDA biosimilar approval of filgrastim-sndz (Zarxio) in 2015, a total of 44 biosimilars for 15 originator products have been approved and more than 20 are marketed.

The oral therapy is available through a REMS program.
This frequency can allow for faster observation of toxicities and adherence issues.
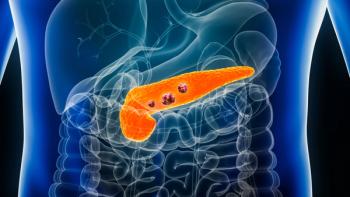
The approval of irinotecan liposome offers an improved first-line treatment option for individuals diagnosed with metastatic pancreatic adenocarcinoma.
This review summarizes current utilization of anti-HER2 targeted therapy across several non-breast solid tumors in the setting of advanced disease.
Repotrectinib led to a durable anti-tumor response in patients with NTRK-positive locally advanced or metastatic solid tumors.
These disorders can be fatal, making pharmacists’ role in detection crucial.

Progression-free survival in the ixazomib/pegylated liposomal doxorubicin/dexamethasone group was shorter than in the ixazomib/lenalidomide/dexamethasone group.
The results indicate promising rates of complete response, manageable adverse events, and dose-dependent exposure in both dose groups.
Treatment options are distinct in their mechanisms, safety profiles, and implications for patients’ quality of life.

In addition to improved progression-free survival, patients treated with this regimen also showed improved complete response or stronger and were minimal residual disease-negative.