
The indication is for symptomatic tenosynovial giant cell tumor (TGCT) for which surgical resection will potentially cause worsening functional limitation or severe morbidity.

The indication is for symptomatic tenosynovial giant cell tumor (TGCT) for which surgical resection will potentially cause worsening functional limitation or severe morbidity.
The combination of the antibody-drug conjugate and PD-1/L1 inhibitor yielded an improved progression-free survival in locally advanced or metastatic urothelial carcinoma.
The data were presented at the 2025 ASCO Genitourinary Cancers Symposium in San Francisco, California.
The cancer cells developed their own electrical network similar to neural behavior seen in the brain.

The expanding role of real-world evidence in regulatory and health technology assessment (HTA) decision-making highlights its growing acceptance for supplementing clinical trial data, addressing evidence gaps, and informing treatment value, despite challenges in data quality, accessibility, and regulatory compliance.
Andrew Lin, PharmD, BCOP, discusses his pharmacist-led study examining the association between early tacrolimus levels and outcomes post-allogeneic hematopoietic cell transplantation.
The combination greatly improved progression-free survival and overall survival.

Talazoparib in combination with enzalutamide yielded significant improvements in radiographic progression-free survival and overall survival.
Currently, the Cadherin-6 targeting antibody-drug conjugate is undergoing evaluation in a phase 1a/1b clinical trial.
Building on a previously granted orphan drug designation, the latest FDA action for amezalpat puts the drug in a position for regulatory approval.
Up to 61% of all non-small cell lung cancer (NSCLC) cases harbor EGFR mutations.

Black women have higher rates of triple-negative breast cancer (TNBC) incidence and mortality than the general US population. Despite this, they are underrepresented in TNBC clinical trials.

Diversity in clinical trials remains important to ensure research data for therapies accurately reflects the diverse patient populations who will use them globally.

The approval follows the ECHELON-3 study, which enrolled adults with relapsed or refractory large B-cell lymphoma.
Molly Schiffer, PharmD, BCOP, discusses the logistical, operational, and financial challenges of delivering cellular therapies in the outpatient setting, focusing on CAR T-cell therapy, TIL therapies, and emerging trends in non-oncology applications.
The approval marks a significant milestone in the treatment of a rare and aggressive subtype of acute leukemia.

Cytopenic myelofibrosis is characterized by poorer prognosis and more severe anemia and thrombocytopenia compared with proliferative myelofibrosis.

The approval is based on positive clinical trial results that indicated deep and durable reductions in plexiform neurofibroma.
Presence of leptomeningeal disease is associated with poorer treatment outcomes and quality of life for patients with breast cancer.



Circulating lactate is increased in patients with myelofibrosis and corresponds with the remodeling of lactate export channel monocarboxylate transporter 4, suggesting a link with fibrosis establishment.
If approved, HLX11 could offer a more cost-effective alternative to pertuzumab for the treatment of human epidermal growth factor receptor 2-positive breast cancer.
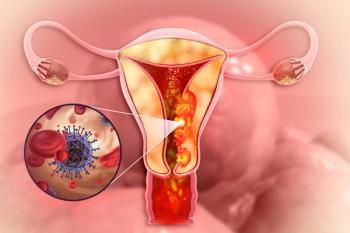
The indication is for patients with endometrial cancer who may benefit from treatment with ACR-368 (Acrivon Therapeutics).

J Ryan Shaw, PharmD, BCPS, BCOP, CPP, discusses the value of the ASTCT and CIBMTR Tandem Meeting 2025 as a platform for multidisciplinary collaboration, cutting-edge education, and advancing pharmacy practice in transplant and cellular therapy.
Zahra Mahmoudjafari, PharmD, BCOP, FHOPA, discusses the logistical challenges and interprofessional strategies essential for delivering cellular therapies in outpatient settings, emphasizing the pharmacist's role in improving patient outcomes and addressing health disparities.
The therapeutic vaccine could provide patients with a non-surgical alternative to traditional treatment approaches.
Molly Schiffer, PharmD, BCOP, discusses the logistical and operational challenges of delivering outpatient CAR T-cell therapy, emphasizing the benefits of outpatient treatment, including reduced costs, improved access to therapy, and better quality of life for patients.

Africa's unparalleled genetic diversity presents both a challenge and an opportunity to advance precision medicine, with a focus on leveraging pharmacogenetics to improve drug efficacy and reduce adverse reactions.

Catriona Jamieson, MD, PhD, discusses the role of stress-activated base editors like ADAR1p150 and APOBEC enzymes in cancer progression and highlights innovative approaches to halt these processes and improve therapeutic outcomes.