
The VENTANA ALK CDx assay is a companion diagnostic to identify patients with ALK-positive non–small cell lung cancer who are eligible to receive treatment with lorlatinib.

The VENTANA ALK CDx assay is a companion diagnostic to identify patients with ALK-positive non–small cell lung cancer who are eligible to receive treatment with lorlatinib.
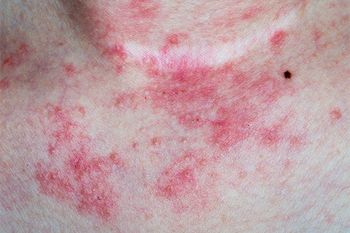
The study authors also mention that samples taken from skin biopsies confirmed their suspicion of a delayed allergic immune response that is commonly seen in drug reactions.
Notably, the United States has now administered the most first-shot COVID-19 vaccinations of any country, with 82.6 million.

Teclistamab showed promising clinical activity and a tolerable safety profile in patients with relapsed/refractory multiple myeloma.
The FDA has issued an emergency use authorization (EUA) for a COVID-19 diagnostic test that can confirm recent or prior COVID-19 infection.
Researchers at Columbia and MIT have created a new technique that can uncover nearly all of the behaviors that cancer cells use to evade immunotherapies, which could lead to the development of more effective treatments.

The guidance said relaxing certain measures for vaccinated individuals may help improve COVID-19 vaccine acceptance and uptake.

Women who attended the 2 more recent screenings prior to their breast cancer diagnosis had a 50% lower incidence of their cancer being fatal within 10 years.

Study findings demonstrate the importance of interventions for multiple sclerosis therapy, such as those supporting medication adherence, adverse effect management, symptom management, and additional counseling.
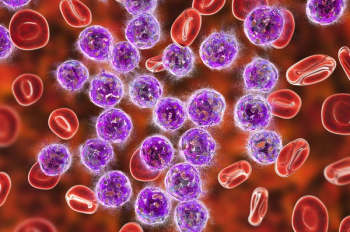
The FDA has approved axicabtagene ciloleucel (Yescarta) for the treatment of adult patients with relapsed or refractory follicular lymphoma following 2 or more lines of systemic therapy.
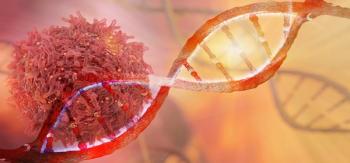
New protein-based immunotherapies could stimulate patients’ immune systems without needing to engineer T cells on an individual basis.

Investigators are set to begin testing sulfasalazine and auronofin, which are FDA-approved to treat rheumatoid arthritis, in cancer cell models.

The FDA has approved a supplemental new drug application for lorlatinib (Lorbrena) to expand the indication to include the frontline treatment of patients with ALK-positive non–small cell lung cancer.
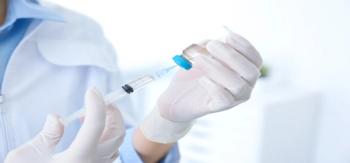
The first vaccine dose alone offered strong protection against COVID-19, with efficacy that did not decline over the 3 months between doses.

President Joseph Biden announced a partnership between Merck and Johnson & Johnson, in which 2 of Merck’s facilities will begin producing the Johnson & Johnson COVID-19 vaccine.

COVID-19 is fueling the expansion of treatment methods beyond medications to include digital therapeutics.

Although there have been many challenges and concerns during the pandemic, experts said there are also lessons to be learned and implemented once the pandemic is over.

Investigators recommended avoiding risk factors such as smoking and switching to a transdermal administration route for testosterone.

Results show respondents' top drivers of happiness in the workplace are colleagues, compensation, and autonomy.
View the full results from the Pharmacy Times 2020 Salary and Job Satisfaction Survey.

The flexibility could allow for easier storage and increased accessibility of the vaccine.

Study findings illustrate the need for better communication between health care providers and patients experiencing concerns during the pandemic.

The investigators said testosterone therapy may have some benefits for older men, but they did not find any benefits in artery function, which is a determinant of future cardiovascular risk.
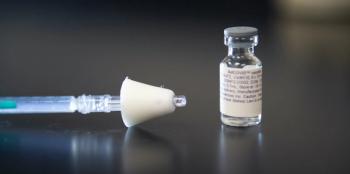
The vaccine has been shown to induce mucosal immunity in the nasal cavity, which may be effective in blocking transmission of SARS-CoV-2.
Pharmacy organizations have produced advisories to guide workflow and optimize services, yet there still exists a gap in further integration of pharmacists into public health and safety initiatives.
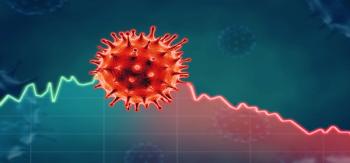
The current surge peaked on January 11, 2021, but took just 5 days to decrease by 10%, compared to 22 days following the first peak in April 2020.

Addressing genomic differences and socioeconomic disparities is essential in order to address the higher incidences and mortality rates for prostate cancer among African American men.

Study findings suggest that enhanced cleaning and disinfecting policies are effective at reducing the spread of COVID-19.
Investigators found an average difference of 12% in the range of fracture-associated drug prescribing when comparing hospital referral regions.

The approval makes adalimumab the only subcutaneous biologic for pediatric patients with ulcerative colitis that can be administered at home.