
Pharmaceutical companies are looking into AI as the new method to not only reduce research and development costs, but also prevent costly errors.

Pharmaceutical companies are looking into AI as the new method to not only reduce research and development costs, but also prevent costly errors.

As part of American Pharmacist Month, Pharmacy Times is asking experts what they believe the value of the pharmacist is.

Previous studies have shown that PAD also impacts health status and functioning.
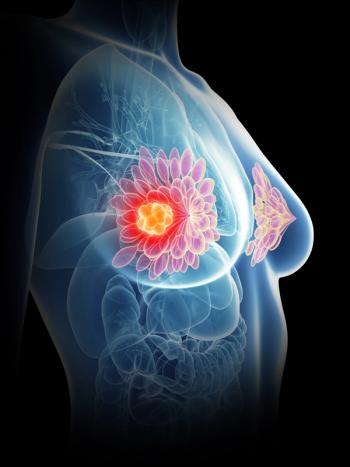
Further, the survey showed that fewer than half of women would flag redness of the breast, thickening of the skin, or 1 breast feeling warmer and heavier than the other as possible symptoms.
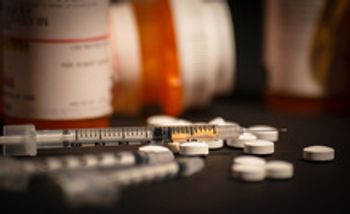
Researchers suggest that tapering a patient’s opioid medications and allowing them to maintain a health care plan can decrease the risk of developing opioid use disorder among other negative outcomes.

Rami Elghandour, chairman and CEO of Arcellx, discussed the company’s investigative CAR-modified T-cell therapy.

Presenters at the AMCP Nexus conference explore outcomes data and whether these medications should be substituted for reference products more frequently.

As a part of the Women in Pharmacy series, Vibhuti Arya, PharmD, MPH, FAPhA, global lead, Gender Equity and Diversity Workforce Development at FIP, discusses moments that shaped her vision for her career in pharmacy.

A presentation at the AMCP Nexus conference focuses on the health care disparities and pharmacotherapy options for gender affirmation.

Presentation at the AMCP Nexus conference focuses on midterm election outlook and a plug for the Pre-approval Information Exchange Act.

This World Standards Week, Carrie Harney, JD, vice president, US Government and Regulatory Affairs at US Pharmacopeia (USP), explains how pharmacists contribute to USP’s standards setting process.

The study will evaluate the safety, tolerability, and efficacy of the in combination in patients both with and without prior exposure to a KRASG12C inhibitor.

As part of American Pharmacist Month, Pharmacy Times is asking experts what they believe the value of the pharmacist is.
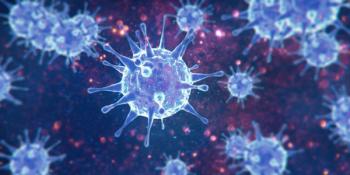
At month 6 post-COVID-19 infection, 123 patients received a diagnosis of type 1 diabetes (T1D) and only 72 were diagnosed with T1D at 6 months post-non-COVID-19 respiratory infection.
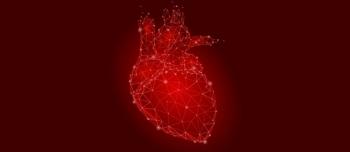
The drug shows improvement in exercise capacity and key secondary outcomes compared with the placebo when added to background therapy for those with pulmonary arterial hypertension.
Investigators found that 25% of patients with heart failure with reduced ejection fraction received guideline-recommended intravenous fluids compared to 37% who did not have the condition.

Keynote speaker at the AMCP Nexus conference discusses recent FDA approvals and those scheduled over the next year.

Jeremy Graff, chief scientific officer at IMV, discusses why the development of viable cancer vaccines is a bit more slow-going in comparison to the fast-paced development of COVID-19 vaccines during the pandemic.
Panelists at the AMCP Nexus conference discuss the burden of undertreated and untreated chronic kidney disease.
Zanubrutinib (Brukinsa) is a small molecule Bruton’s tyrosine kinase inhibitor under evaluation as a monotherapy and in combination with other treatments for various B-cell malignancies.

Experts discuss the clinical and economic impact of misdiagnosing bipolar I disorder as major depressive disorder.

Women who make a difference in the industry honored on #WomenPharmacistDay.
Pharmacists are poised to address selective vaccination and counsel patients contemplating delaying, skipping, or seeking an alternate vaccine schedule.

Heidi D. Finnes, PharmD, BCOP, FHOPA, president of HOPA discusses some key recommendations to women interested in pursuing leadership roles in oncology pharmacy.
The Moderna bivalent vaccine is authorized for children 6 years of age and older, and the Pfizer-BioNTech vaccine is authorized for children 5 years of age and older.

Treating patients with rivaroxaban resulted in 32.5 fewer symptomatic venous thromboembolism (VTE) and VTE-related deaths among 10,000 patients.
Investigators find that individuals with both conditions are more likely to develop severe disease than those in the control group.

Nakia Eldridge, PharmD, MBA, discussed her career, the impacts of COVID-19 on women in pharmacy, and how mentorship can pave the way for more women pharmacists.

As part of American Pharmacist Month, Pharmacy Times is asking experts what they believe the value of the pharmacist is.

This year marks the fifth annual Women Pharmacist Day after it was founded in 2018.