As indicated by prior literature, a second dose of intravenous immunoglobulin (IVIG) in patients with Guillain-Barré syndrome increases serum immunoglobulin G levels without improving clinical outcomes.

As indicated by prior literature, a second dose of intravenous immunoglobulin (IVIG) in patients with Guillain-Barré syndrome increases serum immunoglobulin G levels without improving clinical outcomes.
Factor XI inhibitors show promise as a safer alternative to direct oral anticoagulants for preventing and treating thrombosis, offering similar efficacy with reduced bleeding risk.

Sharita Howe, PharmD, discusses her recent acknowledgement by MM+M, who acknowledged her as a part of the next wave of health care leaders in their recognition of 40 under 40 leaders in health care.
Pharmacists can optimize management of this condition with intravenous iron therapy guidance.
A phase 2 study showed that elotuzumab combined with pomalidomide, bortezomib, and dexamethasone was safe and efficacious.
The 3CL protease inhibitor is designed to suppress the replication of SARS-CoV-2, preventing COVID-19 illness, even if a household member is infected.
The patient is the first to be cured using lovotibeglogene autotemcel, a novel cell-based gene therapy.

In this retrospective study, investigators evaluated a pharmacy-led initiative in the emergency department (ED), finding a potential reduction in ED revisits and hospital readmissions, though results were not statistically significant.

The new indication would include adults with heart failure with a left ventricular ejection fraction (LVEF) of 40% or higher, such as mildly reduced or preserved LVEF.

Although RSV has long been associated with infants and young children, research demonstrates that it is also a significant health risk for adults over the age of 60.
Treatment with ruxolitinib in patients with myelofibrosis and anemia was associated with more inpatient medical center and emergency department visits while increasing health care-related financial burdens compared with nonanemic patients.
Tarlatamab was approved by the FDA in May 2024 for patients who progressed after platinum-based chemotherapy.
This marks steps toward improving health care pricing transparency, but further reforms are needed to reduce costs long-term.
Lipid metabolism plays a critical role in the function of T-cells.
DC646 had a strong safety profile, targeted intestinal FXR, and disrupted FXR-co-activator interactions in metabolic dysfunction-associated steatohepatitis (MASH).

The authors note that further research is needed to clarify cost-effectiveness, long-term adherence, and psychosocial impacts of oral immunotherapy.
The new data emphasize the fundamental differences in management and associated complications with each form of diabetes.
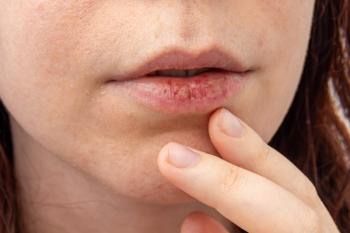
Pharmacists with a deeper knowledge of xerostomia are ideally positioned to help educate patients with dry mouth on making informed self-care decisions for effective symptom management and improved quality of life.
The Western diet was associated with increased risk of lung cancer.
This study found no significant difference in the sustained return of spontaneous circulation or survival to hospital discharge based on the timing of the first epinephrine administration in these patients.
Measures including disease-free survival and overall survival were improved in patients with MF who underwent hematopoietic stem cell transplantation (HSCT) and a 3D volumetric splenomegaly analysis.
Untargeted lipidomics identified a glycerophospholipid and sphingolipid signature that differentiated severe alcohol-related hepatitis (sAH) from decompensated cirrhosis (DC).
Use of dexamethasone over tocilizumab was associated with significant cost-savings for patients with relapsed/refractory multiple myeloma (RRMM).
Compared with daily oral iron, a single intravenous (IV) infusion of iron was shown to lower rates of low birth weight in infants and decrease reliance on further iron supplementation or transfusions in pregnant patients with anemia.

The health care community was left largely unaware of the controversy that occurred at one of the clinical trial sites.
Vepdegestrant is an experimental oral PROteolysis TArgeting Chimera that degrades estrogen receptor (ER) in patients with metastatic breast cancer.
The favorable outcomes were observed in the phase 3 AMPLIFY trial.


