
For the future of precision medicine, many health care providers hope to see a flood of new therapies for biomarker testing to be approved in the next few months.

For the future of precision medicine, many health care providers hope to see a flood of new therapies for biomarker testing to be approved in the next few months.
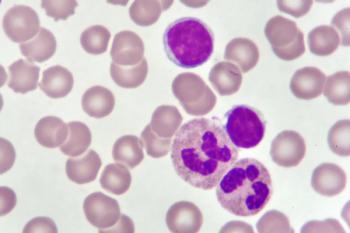
In a presentation at the Community Oncology Alliance 2021 Virtual Conference, Jeff Sharman, MD, summarized the most important developments in care for CLL.
Over the past 18 months, the FDA has approved 4 new regimens for HER2-positive metastatic disease.

The researchers found that patients who reduced their calorie intake by 10% or more and adopted a moderate exercise program immediately after their diagnosis had, on average, 70% less chance of having lingering leukemia cells after a month of chemotherapy than those not on the diet-and-exercise regimen.

In the United States, non–small cell lung cancer accounts for 80% to 85% of all lung cancers, with most patients initially diagnosed with advanced or metastatic disease.

The first step toward operationalizing the uptake of biosimilars is having a program-level discussion that not only describes what biosimilars are, but also assesses how they impact the bottom line and could get incorporated into treatment plans.

The discovery points to a novel treatment target for shrinking brain tumors that arise secondary to breast cancer, according to the study authors.

Pharmacists can lead to the charge to innovate in both 503A and 503B settings, which are poised to change and grow.
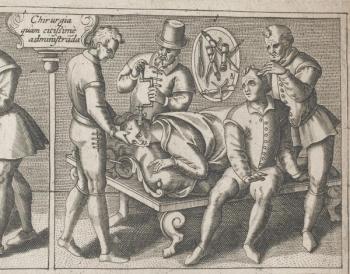
Trepanning is one of the oldest known forms of surgery that was historically used to address issues pertaining to the skull.

Opdivo-based combinations show benefit for patients with esophageal cancer who are often diagnosed after their disease has spread and would benefit from new therapeutic options.

Megan May, PharmD, BCOP, Cecilia Lau, RPh, BCOP, APh, and Daneng Li, MD, share their thoughts on unmet needs in the management of neuroendocrine tumors.

A session at the Community Oncology Alliance virtual 2021 conference noted that clinical trials have found promising new treatments for patients with breast cancer, offering opportunities for future new approvals and indications.

Developing an antimicrobial stewardship program is arduous but valuable; commitment from leadership is the first step.

In patients who received early palliative care, researchers noted a lower percentage of patients with depression, improved quality of life, and, significantly, longer survival.

Panelists at the Community Oncology Alliance (COA) virtual 2021 conference said the impacts of the pandemic on their day-to-day operations were challenging, although they all said they were proud of how their staff handled the rapid changes in spring of 2020.

Findings from the phase 3 ASCENT trial demonstrate a clinically meaningful 57% reduction in the risk of disease worsening or death for patients receiving sacituzumab govitecan.
Patients with glioblastoma receiving temozolomide in the morning had an average overall survival of about 17 months, compared to an average overall survival of approximately 13.5 months for those taking the drug in the evening.
Although many attendees and presenters are looking forward to returning to in-person conferences in the future, Anne Krolikowski said the virtual format of their upcoming conference allows even more people to attend.

Staying educated in the formal and informal health care settings surrounding state-of-the-art cancer care in the community practice is essential for success.

Based on their findings and considering the small size and portability of the test, the investigators said it could be used for fast virus detection in schools and airports.
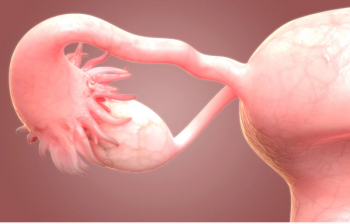
Study indicates patients with ovarian cancer frequently receive aggressive end-of-life care despite industry guidelines that emphasize quality of life for those with advanced disease.

According to previous studies, youth who are obese when they begin chemotherapy are more than twice as likely to have remaining cancer cells after 1 month of treatment compared to their lean counterparts.
Empiric anti-MRSA therapy is an area of opportunity for antimicrobial stewardship in the treatment of CAP.
As hematology and oncology pharmacists have become increasingly essential members of the care team, new investigational drugs could have a significant impact on the treatment landscape.
Two-thirds of surveyed physicians said patients are presenting with more late-stage cancers, with 73% saying patients in their practice are not receiving screenings.

Guidelines typically recommend debulking surgery, plus systemic chemotherapy for most patients.

Health System Owned Specialty Pharmacy Alliance issues open letter outlining three needed industry actions to improve patient care and outcomes.

The oncology space has seen rapid growth in the last decade, with new approvals and indications necessitating expertise in areas such as medication use, safe practices, and reimbursement practices.
Hager Hanon, PharmD, RPh, senior pharmacist at Community Care Rx, discusses COVID-19 vaccination hesitancy among pregnant women and people with a history of anaphylaxis.
Further, the findings indicate that rapid screens can predict infection with nearly the same precision as antibody tests conducted in a lab, which could be useful for providers.