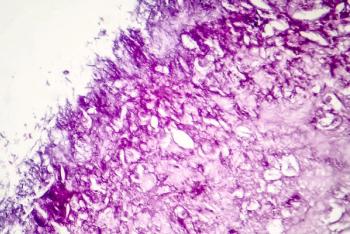
The FDA has granted Orphan Drug Designation (ODD) to alrizomadlin (APG-115, Ascentage Pharma) for the treatment of stage IIB-IV melanoma.

The FDA has granted Orphan Drug Designation (ODD) to alrizomadlin (APG-115, Ascentage Pharma) for the treatment of stage IIB-IV melanoma.
Investigators have found that a small percentage of epidermal growth factor receptor (EGFR) inhibitor-resistant cancer cells are present in certain patients with non-small cell lung cancers (NSCLCs), potentially explaining tumor regrowth in these patients.

Because there are no other available treatment options, Pamela Spicer said the approval of aducanumab gives much-needed hope to patients with Alzheimer disease and their families.

The investigators said the ACA helped lower the financial burden for younger cancer survivors to its lowest estimated levels in 20 years.

Understanding the categories is key to evaluating and making decisions about outsourcing products or improving internal production standards.
The FDA has approved pembrolizumab in combination with lenvatinib for the treatment of advanced endometrial carcinoma that is not microsatellite instability-high or mismatch repair deficient

Although she said it is unlikely that the FDA will retract the approval, prescribers could be reluctant to administer aducanumab to patients.
The results indicated that T cell responses—including CD8+ T cells, which seek out and destroy infected cells—persisted over the 8-month timeframe of the study, according to the authors.
Venetoclax is already approved for the treatment of adults with chronic lymphocytic leukemia or small lymphocytic lymphoma, and in some adults with newly diagnosed acute myeloid leukemia.
Patients treated with berubicin in clinical trials appeared to demonstrate positive responses, including 1 durable complete response in a phase 1 human clinical trial.
Pharmacy Times interviewed Craig Freyer, PharmD, BCOP, and Andrew Lin, PharmD, BCOP, to discuss the emerging data around BCMA-targeted CAR T-cell therapy in multiple myeloma.

In addition to concerns about the data supporting the FDA approval of aducanumab for patients with Alzheimer disease, FDA advisory committee members raised some unique concerns when they rejected the application in November 2020.
Many HTCs use these drug pricing arrangements to reduce reliance on limited federal funding.

The FDA has approved the first and only intravenous immunoglobulin indicated for use in adults with dermatomyositis.
Study findings could offer a new approach to the toxin-based approach of other C. difficile vaccines in development.

Despite their importance, formal performance appraisal systems are often lacking.

The drug is an investigational oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI) for patients with anemia due to chronic kidney disease (CKD).
Zydelig (idelalisib) is a kinase inhibitor approved in 2014 for 3 oncology indications.

Despite the FDA's approval of aducanumab for the treatment of Alzheimer disease with mild cognitive impairments, it has been surrounded by questions.

Trial identifies no new safety signals, as emicizumab continues to demonstrate effective bleeding control with a high proportion of participants achieving zero treated bleeds.
PBMs can implement a clinically rigorous approach to specialty pharmacy management that ensures members have appropriate access while helping payers control their pharmacy spend.
Cabozantinib (Cabometyx, Exelixis) is a kinase inhibitor approved for indications such as advanced renal cell carcinoma and hepatocellular carcinoma.

Clinicians cite long-term complications and younger patients with better prognoses as reasons to pull back on oropharyngeal cancer therapy.

The FDA has approved finerenone for the treatment of adult patients with chronic kidney disease associated with type 2 diabetes.

The 22F and 33F serotypes, which are associated with high rates of invasiveness and antibiotic resistance, are unique to the pneumococcal 15-valent conjugate vaccine.

The research team analyzed data from the Household Pulse Survey in March 2021, which is an online nationally representative sample of the population conducted by the US Census Bureau and the National Center for Health Statistics and other agencies.
Pharmacy Times interviewed Craig Freyer, PharmD, BCOP, a clinical pharmacy specialist at Penn Medicine in the University of Pennsylvania Health System, and Andrew Lin, PharmD, BCOP, an oncology pharmacy specialist at Memorial Sloan-Kettering Cancer Center, to provide an overview of current CAR T-cell therapies.

The FDA has granted Fast Track Designation to trilaciclib (Cosela) for use in combination with chemotherapy for the treatment of locally advanced or metastatic triple-negative breast cancer (TNBC).
Institute for Safe Medication Practices advocates technology solutions, such as barcode scanning of ingredients
Findings from the 2 pivotal phase 3 trials had mixed results on cognitive benefit, although they found a positive reduction in amyloid plaque in patients' brains.