Approved indications for idelalisib include relapsed chronic lymphocytic leukemia, small lymphocytic lymphoma, and follicular B-cell non-Hodgkin lymphoma.

Approved indications for idelalisib include relapsed chronic lymphocytic leukemia, small lymphocytic lymphoma, and follicular B-cell non-Hodgkin lymphoma.

Study findings could influence how drug developers consider how opioid drugs cause tolerance and respiratory depression, and it suggests an alternative approach to developing safer analgesics.

Although the average demand for tocilizumab increased by 279% during the study period, fill rates for orders by hospitals dropped from 99% to 45%.
The data represent teens most likely to benefit from the vaccination and are meant to offer reassurance to parents and clinicians, even with small study numbers.
OSA is associated with cardiovascular disease in adults, but less is known about how the condition affects the immediate and long-term heart health of children and adolescents.

Relugolix is an orally administered treatment for prostate cancer that works by blocking the pituitary gland from making luteinizing hormone and follicle-stimulating hormone.

This is the first time that the intravenous (IV) formulation of brivaracetam will be available for pediatric patients and is the only IV formulation approved for partial-onset seizures and this age group in nearly 7 years.
Previous studies have demonstrated an association between low levels of vitamin D in the blood and pain, sensitivity to infection, fatigue, depression, and lower self-rated quality of life.

This designation could shorten the FDA review period to 8 months compared to the 12 months under Standard Review.
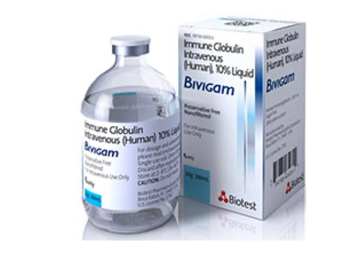
Immune Globulins are injections that are used to treat immunodeficiency disorders.

Brad McElya, PharmD, director of specialty health solutions at Walgreens, discusses the critical role pharmacists play in providing HIV prevention drugs in communities across America.
The kinase inhibitor is a promising treatment option for patients with MET exon 14 skipping mutation non–small cell lung cancer.

Silmitasertib has produced clinical benefit as a monotherapy and in combination with drugs such as gemcitabine and cisplatin.

For the study, approximately 20,000 people were followed over a span of 4 years, which showed that reports of marijuana use peaked at 9% for patients with cancer versus 14% among those with no cancer history.
The treatment was also well-tolerated, with a similar safety profile to placebo, according to the investigators.

The approval of Tibsovo marks the first and only targeted therapy approved for patients with previously treated IDH1-mutated cholangiocarcinoma.
At 30 days following the third dose in the series, the pneumococcal 15-valent conjugate vaccine was non-inferior to the 13-valent vaccine for all 13 shared serotypes.

Pharmacists must remain nimble to help patients continue receiving the medications they need, as technology now plays a central role in workflow and medication management.

Tisagenlecleucel is a CD19-directed genetically modified autologous T cell immunotherapy already indicated for B-cell precursor acute lymphoblastic leukemia and diffuse large B-cell lymphoma.

Between 40% and 50% of patients respond long term without relapsing.

SARS-CoV-2 neutralizing titers against the wild-type strain one month after the booster dose were 3.3 times higher than the titers found one month after the second dose.
Johnson & Johnson previously reported interim results from a phase 1/2a trial demonstrating the antibody responses generated by their vaccine were strong and stable through 8 months following immunization, published in the New England Journal of Medicine.

A final blinded analysis of data from the phase 3 COVE study found 93% efficacy of Moderna’s COVID-19 vaccine, which remained durable through 6 months after the second dose.

With more work and less staff, we need to examine how we can better support pharmacists in the new normal brought on by COVID-19.
As the price tags for therapies coming to market have soared into the hundreds of thousands and even millions on occasion, paying for them has become an ever-greater cause of concern and focus area.
However, according to the investigators, eradicating COVID-19 is considerably less feasible than the elimination of smallpox.
Researchers found that the levels of serum albumin and the number of metastatic sites that patients with lung cancer have were significantly associated with overall survival.
The study, conducted by Augusta University, was among the first to examine factors related to vaccine hesitancy among a large African American community sample, according to the authors.

Active surveillance, intended to avoid unnecessary treatment and the resulting adverse effects, typically involves regular prostate-specific antigen (PSA) screenings, prostate exams, imaging studies, and repeat biopsies to carefully monitor prostate cancer growth or progression without compromising long-term outcomes.
Cholangiocarcinoma is a rare bile duct cancer, with physicians diagnosing approximately 8000 cases each year.