
The companies have decided to unblind the trial and perform no additional analyses for the OS endpoint because there was no clinical benefit observed in the doublet therapy arm.

The companies have decided to unblind the trial and perform no additional analyses for the OS endpoint because there was no clinical benefit observed in the doublet therapy arm.
The findings, published the New England Journal of Medicine, showed treatment with rilzabrutinib led to a rapid, durable increase in platelet count while supporting an acceptable safety profile.
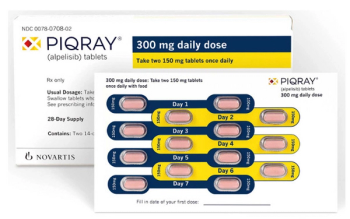
Piqray is indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with HR-positive/HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer, as detected by an FDA-approved test following progression on or after an endocrine-based regimen.

Sarah Wheeler, PharmD, BCOP, clinical pharmacy specialist in Hematology/Oncology at UF Health Shands Cancer Hospital, discusses how experience as a caregiver can help inform the work of an oncology pharmacist on the clinical care team.

Sarah Wheeler, PharmD, BCOP, clinical pharmacy specialist in Hematology/Oncology at UF Health Shands Cancer Hospital, discusses how oncology pharmacists with experience as a caregiver can utilize those skills while still honoring the boundaries of both themselves and their patients.

The first part of a series outlining 5 crucial steps so pharmacies can enhance patient engagement and lower operational costs.

Sarah Wheeler, PharmD, BCOP, clinical pharmacy specialist in Hematology/Oncology at UF Health Shands Cancer Hospital, discusses some of the unique struggles that come from being an oncology pharmacist and a caregiver.

Sarah Wheeler, PharmD, BCOP, clinical pharmacy specialist in Hematology/Oncology at UF Health Shands Cancer Hospital, discusses how oncology pharmacists can help their patients overcome some of the barriers patients with cancer may encounter during treatment.

Lack of acknowledgement or rewards in high-pressure pharmacy environments can lead to mental health issues.

Benyam Muluneh, PharmD, BCOP, CPP, assistant professor at the University of North Carolina Eshelman School of Pharmacy, discusses his presentation at the HOPA 2022 annual conference on the Oral Chemotherapy Collaborative (OCC) and how it works to optimize care for patients taking oral anti-cancer agents.

Cabotegravir is the only current HIV-1 PrEP medication that does need to be taken daily.

The companies plan to submit these data to the FDA in the coming days for an Emergency Use Authorization of a booster dose for children ages 5 through 11 in the United States.
Vusolimogene oderparepvec is in development for the treatment of multiple skin cancers either alone or in combination with anti-PD1 therapy.

Patient’s lawsuit alleges 4 legal theories as bases for liability, including defamation and emotional distress.
Because there is an inflammatory component to Down syndrome regression disorder, it may potentially be reversible with immunotherapy.
Ponesimod (Ponvory) is indicated for the treatment of relapsing forms of multiple sclerosis.

The investigational selective, covalent, and orally bioavailable KRASG12C¬ inhibitor shows a 57% confirmed overall response rate at the recommended dose of 200 mg twice daily.
Pharmacists should review the medications of patients with multiple sclerosis regularly to assess drug-drug interactions.
The FDA previously granted Fast Track Designation for the combination of lifileucel and pembrolizumab in metastatic melanoma.

Relevant training and credentials lead to technicians taking a more active role in pharmacies.

Based on event-free survival and pathologic complete response results, the FDA approved nivolumab in combination with platinum-doublet chemotherapy in March 2022.
Results are from an exploratory analysis of 34 patients who received at least 1 dose after disease progression or suboptimal response with ruxolitinib monotherapy
Results show that 64% of individuals treated with lorlatinib were without disease progression after 3 years compared with about 19% of those treated with crizotinib (Xalkori).

Blue Earth Therapeutics will begin an open-label, multicenter trial to evaluate a next-generation radiopharmaceutical for metastatic castration-resistant prostate cancer.
It is imperative for health care professionals, especially pharmacists, to be well-informed regarding the physiological impact of Ramadan on diabetes.
Clinical data from phase 1/2 LIBRETTO trial show evidence of meaningful clinical outcomes for patients, including those with difficult-to-treat brain metastases.
Sotorasib demonstrates clinical benefit and prolonged tumor response seen, with a 40.7% objective response rate.
Ravulizumab-cwvz (Ultomiris) produced statistically significant improvements in measures of functional activity, muscle strength, and quality of life in patients with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis.

As job duties continue to change, having coverage protects against alleged errors or omissions.

Biosimilars could save consumers more than $100 billion in the coming years.