
The increase in gabapentin-related overdose deaths follows a rising trend in overall overdose deaths during the COVID-19 pandemic.

The increase in gabapentin-related overdose deaths follows a rising trend in overall overdose deaths during the COVID-19 pandemic.

The FDA granted the vaccine series Emergency Use Authorization on July 14, based on clinical trial data showing significant efficacy and safety.
Poziotinib is an irreversible pan HER2 inhibitor with activity against mutations of HER1, HER2, and HER4.
Approximately one-third of the individuals had an intracranial response among those with central nervous system metastases.
For coinfected patients who first contract influenza A, replication of SARS-CoV-2 may be suppressed, even 1 week after clearance of influenza A.
Individuals vaccinated simultaneously with COVID-19 booster and influenza vaccines may be more likely to experience systemic reactions and adverse health impacts.

Dronabinol (Syndros) is a cannabinoid indicated in adults for the treatment of: anorexia associated with weight loss in patients with AIDS and nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments.

Companies that incorporate Diversity, Equity, and Inclusion (DEI) into their everyday operations will be instrumental in shaping a new standard in their workforce and ultimately in society.
Analysis shows that the common infection may be a driver of the disease in adults younger than aged 50 years.
Elecsys HCV Duo allows independent and simultaneous determination of early-stage infection and those who are recovering or showing signs of a chronic problem.
What we're seeing as a trend is this significant increase in synthetic opioids, specifically fentanyl, being contaminated and causing severe overdoses.

Donald Klepser, PhD, MBA, professor and dean of academic affairs at the College of Pharmacy in the University of Nebraska Medical Center, discusses how pharmacy technicians can manage their own stress and burnout.
This inflammation of the colon poses a substantial burden to patients and the health care system.

Quality refers to a set of systems that minimizes errors, maximizes performance and efficiency, and which provides the user with an optimal experience to receive the greatest health benefits.

Hyqvia is an immune globulin with a recombinant human hyaluronidase indicated for the treatment of primary immunodeficiency in adults.

Indications for adalimumab include ankylosing spondylitis, Crohn disease, chronic plaque psoriasis, juvenile idiopathic arthritis, moderate to severe rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.

Patients at highest risk of cancer recurrence, including people with lymph node-positive disease, saw the greatest reduction in the risk of recurrence or death with the pertuzumab-based regimen

Organizations such as the Appalachian Community Cancer Alliance are working to leverage technology and improve barriers to care.
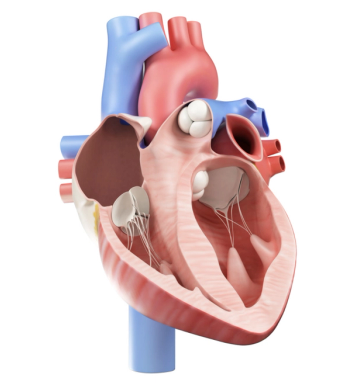
Results are first to show causal relationship between losing mLOY in the blood and development of cardiovascular disease.

Data indicate that consumption of 7 or more units of alcohol per week is associated with higher iron levels in the brain, which has been linked to Alzheimer and Parkinson diseases.

This month, we spoke with Bob Luschen, PharmD, clinical coordinator for the pharmacy department at cancer Treatment Centers of America, Atlanta.
Dalbavancin (Dalvance) is indicated for acute bacterial skin and skin structure infections caused by designated susceptible strains of Gram-positive microorganisms.

Health system specialty pharmacies help remove barriers to care, optimize therapy, and improve outcomes.
Pharmacists can leverage patient medical history for formulating effective drugs, and patients can also benefit from single dosage, greater efficacy, and affordability of compounded medications.

Data from Roche’s phase 3 HAVEN 6 study show clinically meaningful bleed control, with no new safety signals observed.

Influenza viruses typically circulate in the Northern Hemisphere during the winter and spring, but they occasionally can circulate more during the summer months, as did the 2009 H1N1 pandemic strain.

Building a team to support best practices, medication safety, and staff education will reduce risks.

Ryan Burke, PharmD, director of professional affairs at Pharmacy Technician Certification Board, discusses how the changing role of pharmacy technicians has also helped the role of the pharmacist.
Patients with diabetes on Medicare Advantage were also less likely to be prescribed newer, more expensive medicine than patients on Medicare Fee-For-Service plans, indicating potential disparities in care.
Patients with heart failure in bereavement are at a higher risk of death from the condition, particularly in the first week following a family member’s death.