
Joe DePinto discusses innovative payment models like value-based agreements and warranties for cell and gene therapies

Joe DePinto discusses innovative payment models like value-based agreements and warranties for cell and gene therapies
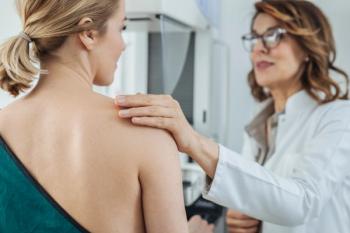
The screenings should be conducted every other year, beginning at age 40 and continuing through age 74, according to the new recommendation.
Sarah Butler discusses the important role pharmacists play in educating patients on these changes in medication prices.
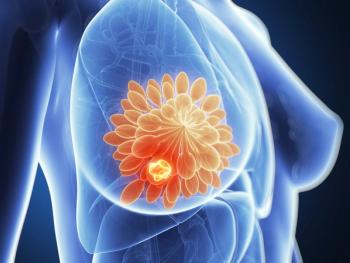
The investigators note that additional research is needed to confirm positive overall survival trends in this patient population.
The 1-time treatment may be a better alternative for patients who don’t want to undergo frequent doses of standard of care intravenous factor IX infusions.

Prevention of acute cardiac events may be another consideration for future postlicensure studies of RSV vaccines.

Vitamin D deficiency (VDD) is an important factor in the pathogens of schizophrenia.

Pharmacists can help patients distinguish between food allergies and intolerances, as well as facilitate access to life-saving medications and preventative care.

Christie Smith discusses a medically integrated dispensing model that aligns the goals of patients, physicians, and pharmacists.

HSSPs improve medication adherence and reduce total health care costs by directly managing medications for complex chronic conditions like cancer, diabetes, COPD and CHF.

Trastuzumab is indicated for adjuvant breast cancer, metastatic breast cancer, and gastric cancer.

Vincent Young, MD, PhD, shares takeaways from Peggy Lillis Foundation's 2024 National C diff Advocacy Summit.

The CEO and co-founder of the Peggy Lillis Foundation discusses highlights from the organization's 2024 National C diff Advocacy Summit.
Tovorafenib is the first systemic therapy to be approved for the treatment of pediatric patients who have low-grade glioma with BRAF rearrangements or fusions.

Shawn Riser Taylor, PharmD, CPP, CDCES, discusses the challenges and process of providing health care in a rural area of Guachipilincito in Honduras.
The approval is based on data from Study 5310, evaluating the pharmacokinetics, safety, and efficacy of Biktarvy (Gilead Sciences Inc) in pregnant individuals.

On this month's episode Laura Gillespie, PharmD, discusses her paper “Impact of Pharmacist-Led Initiatives on Health Care–Associated Clostridioides difficile Rates.”
The severity of RSV disease among adults further highlights how crucial vaccine polices and recommendations are.

The findings emphasize a need for guideline-based care delivery for patients with severe asthma, particularly for patients who are facing social disparities within health care.

Dostarlimab plus chemotherapy is the only immuno-oncology-based therapy that showed statistically significant and clinically meaningful survival benefit in the overall patient population.
A 76% lower risk was displayed with adjuvant alectinib compared to chemotherapy treatment for non–small cell lung cancer.
Iptocopan demonstrated superiority when treating patients with immunoglobulin A nephropathy and is the first drug to specifically target the alternative complement pathway.

Pivmecillinam (Pivya; UTILITY therapeutics Ltd) tablets were approved for female adults with uncomplicated urinary tract infection caused by Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.
The recombinant zoster vaccine demonstrated a 79.7% efficacy in patients aged 50 years and older cumulatively within the 6- to 11-year period after vaccination.

Laura Gillespie, PharmD, discusses some of the financial benefits of having a pharmacist focused on antimicrobial stewardship efforts in a health system.

Exploring challenges and opportunities in developing personalized treatments for schizophrenia
The approval was based on the QUILT-3.032 study, which included 77 adults with carcinoma in situ with or without papillary tumors after a transurethral resection.
Frexalimab demonstrated a favorable tolerability profile after approximately 1 year of treatment for individuals with relapsing disease.
Ofatumumab (Kesimpta; Novartis) demonstrated a sustained efficacy as a first-line, continuous treatment for patients recently diagnosed and treatment-naïve with relapsing multiple sclerosis.