
New research confirms the amyloid hypothesis as a major pathophysiologic factor in the development of Alzheimer's disease.

New research confirms the amyloid hypothesis as a major pathophysiologic factor in the development of Alzheimer's disease.
New research raises questions about the efficacy of one of the most commonly prescribed drugs used to treat relapsing-remitting multiple sclerosis.
Research on epithilone D, an investigational drug targeting the formation of tau tangles in Alzheimer's disease, was presented at the 2012 Alzheimer's Association International Conference in Vancouver, British Columbia.
The FDA approved Truvada for preexposure prophylaxis, making it the first agent ever to be approved for HIV prevention in uninfected adults.

New legislation recently signed by the president promises to get novel therapies to patients in a more timely manner through the FDA's Accelerated Approval process.

Legislation by representatives Michael McCaul (R, TX) and G.K. Butterfield (D, NC), offering drug companies millions of dollars in incentives to develop treatments for rare pediatric disease, was recently signed by the president.
A new study finds that patients with autoimmune diseases being treated with immunosuppressive drugs are not at an increased risk of developing shingles.

Specialty Pharmacy Times spoke to Milayna Subar, MD, vice president and national practice leader in oncology for the Oncology Therapeutic Resource Center (TRC) at Express Scripts, to learn more about how access to clinical practice guidelines in oncology can help shape the complex landscape of cancer treatment.

The FDA today approved OraQuick In-Home HIV Test, the first over-the-counter HIV test that gives users results without having to send samples off to a lab.

To get perspective on the ACA ruling's impact on specialty pharmacy, Specialty Pharmacy Times interviewed D'vorah Graeser, PhD, about the Biologics Price Competition and Innovation Act (BPCIA).

June 27th is National HIV Testing Day, an annual campaign coordinated by the National Association of People with AIDS to encourage people of all ages to "Take the Test, Take Control."

A recent FDA study determined that current nucleic acid testing (NAT) of the plasma of donated blood is adequate for the detection of the hepatitis C virus (HCV).
Sanofi's Genzyme unit announced in a press release earlier last week that the company has submitted an application to regulatory authorities for approval of Lemtrada (alemtuzumab) for the treatment of relapsing multiple sclerosis (RMS).

Diplomat Specialty Pharmacy CEO Phil Hagerman was recently invited to participate in a forum on fostering greater opportunity, innovation, and job creation in today's economy.

On June 20th, 2012, the longest day of the year, the Alzheimer's Association is asking participants to run, walk, bike, or engage in some kind of endurance activity for 16 consecutive hours to honor those facing Alzheimer's disease.
The FDA will take an additional 3 months to review the drug application for Truvada as a preventive HIV treatment to account for updated safety materials submitted by Gilead.

Testing baby boomers could help identify more than 800,000 new cases of hepatitis C and address the largely preventable consequences of the condition.

Bristol-Myers Squibb's investigational drug facilitates improved immune response in lung cancer, kidney cancer, and melanoma patients, according to a study presented at the American Society of Clinical Oncology (ASCO).
Girls who received mantle radiation for childhood cancer were found to be at elevated risk for secondary breast cancer later in life.
Afatinib shows promise as a treatment for patients with advanced lung adenocarcinomas who tested positive for EGFR mutations.

A new single agent called dabrafenib for the treatment of metastatic melanoma appears to significantly slow progression of the disease, according to reports from the American Society of Clinical Oncology (ASCO) meeting.

Employers are concerned with worker health behaviors and want to control spending on expensive specialty medications, according to a new survey released by Express Scripts.

Testing this sample of the population could help identify more than 800,000 new cases of hepatitis C and address the largely preventable consequences of the condition.
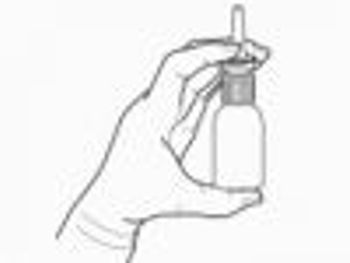
Researchers will receive $7.9 million over the next 5 years to test the effects of intranasal insulin therapy on patients with mild cognitive impairment and mild to moderate Alzheimer's disease.

The plan marks the creation of a landmark national strategy to increase public awareness of Alzheimer's disease.

Patients with certain preexisting heart conditions, history of stroke, or those who are taking antiarrhythmic medications are being advised to use the oral MS agent Gilenya cautiously.
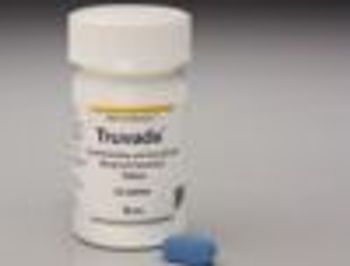
An FDA advisory panel has recommended Truvada as a pre-exposure prophylaxis for people at risk of contracting HIV.
The committee voted 8-2 to recommend approval of the investigational agent tofacitinib, and an FDA decision could come as early as August.
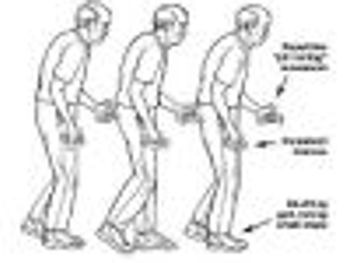
Modifying the activity of the gene that becomes mutated in Parkinson's disease may alter disease progression.
More than 75 million dollars worth of stolen medications was recently recovered from a Florida warehouse.