Sanofi slashed the price of colorectal cancer drug Zaltrap (ziv-aflibercept) in half after 2 oncologists from Memorial Sloan-Kettering Cancer Center publicly balked at the drug's $11,000 price tag in an op-ed in The New York Times.

Sanofi slashed the price of colorectal cancer drug Zaltrap (ziv-aflibercept) in half after 2 oncologists from Memorial Sloan-Kettering Cancer Center publicly balked at the drug's $11,000 price tag in an op-ed in The New York Times.

Value-added services for specialty drugs can help distributors support otherwise slim profit margins.

Proposed legislation would allow the FDA to track the volume and distribution of drugs produced at compounding pharmacies, provide more information to the public about compounded drugs, and institute a waiver system in times of public need.

New legislation would allow the FDA to track the volume and distribution of drugs produced at compounding pharmacies, increase the transparency of compounded drugs, and institute a waiver system in times of public need.

Considering the delicate nature of specialty pharmaceuticals, specialty pharmacies must take measures to ensure they have a crisis communication plan in place in the event of a natural disaster or an emergency.

The American Society of Clinical Oncology's list of the top 5 common oncology treatments or procedures that "lack sufficient evidence for widespread use" should be considered when discussing the rising cost of cancer care.
In a session at the 2012 Academy of Managed Care Pharmacy Educational Conference, Aimee Tharaldson, PharmD, identified the top specialty drugs in the pipeline and highlighted the most recent therapeutic trends within each medication class.
A new study carried out by Blue Cross and Blue Shield of Minnesota and PBM Prime Therapeutics forecasts that multiple sclerosis specialty drug treatment cost will exceed $50,000 per person per year in 2016.
A recent outbreak of meningitis tied to a compounded drug raises concerns about the scope of the practice of pharmacy compounding and prompts legislators to push for increased FDA authority over these products.
Therapies for chronic conditions are increasingly being subjected to utilization management tools, according to ICORE Healthcare's 2011 Medical Pharmacy & Oncology Trend Report.
Humira, Abbott's biggest seller, was recently approved by the FDA to treat ulcerative colitis.
Study of the biology of squamous cell lung carcinoma leads a team of scientists to conclude that potential new cancer treatment approaches should focus on genetic aberrations rather than on the organ of origin.

Patients taking Truvada may still significantly reduce their risk of HIV infection even when they are not 100% adherent to their drug regimens, a new study revealed.
When compared with traditional treatments, biologic therapies for RA were found to not be associated with an increased risk of cancer.

A new report finds that among all the types of physicians, oncologists are most likely to restrict visits with pharmaceutical reps.

The lifetime revenue potential of orphan drugs far exceeds the financial potential of other medications, according to a recent report conducted by researchers from Thomson Reuters Life Sciences Professional Services and Pfizer.
Breakthrough cancer pain-episodes of intense pain experienced by many cancer patients despite around-the-clock treatment with opioids-can be effectively managed with a fentanyl-based oral spray.

Although many of the anticompetitive claims levied against the ESI-Medco merger did not survive a recent United States District Court ruling, the claim regarding the sale of specialty pharmaceuticals remains intact.

Stribild, previously known as the Quad, has been approved by the FDA to treat HIV-1 infection in adults who are starting HIV treatment for the first time.
Scientists show a renewed interest in latrepirdine as a treatment for a number of neurodegenerative disorders after experiments with the drug exhibited improved memory function in animal models.
The business and science of innovation are challenged by a recent article in British Medical Journal entitled "Pharmaceutical research and development: what do we get for all that money?"

Dr. D'vorah Graeser weighs in on the increasingly popular practice of using pay-for-delay arrangements to settle disputes over pharmaceutical patent ownership.
The new platform provides patients with a reliable place to go for information about their disease state and specialty medications.
Through their studies of the molecular mechanisms involved in DNA repair, scientists from Case Western Reserve University School of Medicine found that the artificial activation of a gene called Chk1 permanently halts cancer cell replication.

Fresh on the heels of another failed drug trial, pharmaceutical companies try to gauge the remaining strategic options in the treatment of patients with Alzheimer's disease.
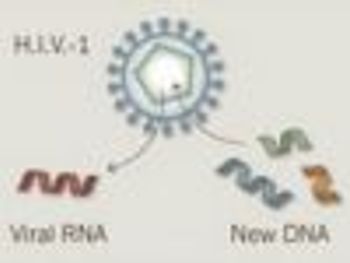
A new research and licensing partnership between the University of Pennsylvania and Novartis aims to develop more effective treatments for cancer by giving patients a disabled form of HIV.

A provision of the new health care law expands womens' access to potentially life-saving tests and services.

Treatment with antiretrovirals is now recommended for all adults with HIV infection, regardless of their CD4 count.

Specialty Pharmacy Times spoke to Sajid Syed, BSPharm, MS, RPh, of Acro Pharmaceutical Services, about the high cost of specialty drugs, patient needs, and the ACA.
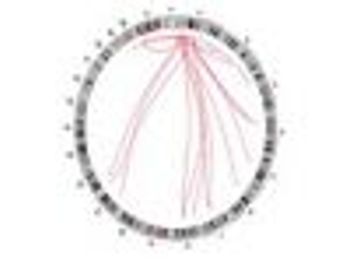
NCI's research uncovers new potential drug targets and pathways that may someday be incorporated into new therapeutic strategies.