Innovative digital twin technology revolutionizes brain cancer treatment by predicting tumor metabolism and personalizing therapies for better patient outcomes.

Danielle Valletti is an assistant editor at Pharmacy Times®. She graduated from The Pennsylvania State University in May 2025 in journalism and media studies. Prior to this position, she worked as a reporter and editor for a digital news organization.

Innovative digital twin technology revolutionizes brain cancer treatment by predicting tumor metabolism and personalizing therapies for better patient outcomes.
Explore the differences between oral and injectable semaglutide for weight management, focusing on adherence, adverse effects, and patient preferences.

New research reveals a gut bacteria-derived molecule that enhances lung cancer immunotherapy, potentially improving outcomes for patients with limited treatment options.

Pitavastatin shows promise as a novel treatment for triple-negative breast cancer, enhancing chemotherapy efficacy and targeting cancer cell survival pathways.

Pharmacists play a vital role in mental health care, enhancing treatment continuity and bridging gaps between mental and physical health for patients with serious mental illness.

The FDA approves Naox Link, a groundbreaking in-ear EEG device, transforming brain monitoring and enhancing neurologic care for patients and pharmacists.

Recent studies reveal that weight recovery in anorexia nervosa does not ensure muscle restoration, highlighting the need for comprehensive recovery strategies.
New dietary guidelines emphasize higher protein intake, reduced added sugars, and avoidance of ultra-processed foods to combat chronic diseases.
The role of cancer-associated fibroblasts in non–small cell lung cancer (NSCLC) drug resistance highlights innovative strategies to enhance treatment efficacy.

Explaining megestrol acetate's dual role in managing symptoms and enhancing treatment efficacy for estrogen receptor–positive breast cancer.

Toothpaste tablets revolutionize oral care with eco-friendly, convenient options, while pharmacists guide consumers in making informed choices for dental health.

GoodRx enhances access to semaglutide with affordable cash pricing, revolutionizing obesity treatment and supporting patients in weight management.

FDA considers relaxing dietary supplement warning labels, raising concerns about consumer safety and informed decision-making among users.

Expert highlights the critical need to manage skin toxicities in oncology, ensuring effective cancer treatments while addressing patient well-being and adherence.

New research links ultra-processed foods, especially processed meats, to higher breast cancer mortality among Black women, highlighting dietary changes for better outcomes.
New findings on nodal radiation for breast cancer patients highlight the importance of lymph node involvement and tumor size in treatment decisions.
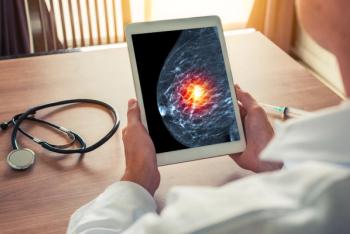
The FLEX study enhances breast cancer treatment by integrating genomic data, guiding therapy choices, and improving patient outcomes.

Discover the latest FDA-approved treatments, including lumateperone for depression and elinzanetant for menopause symptoms, plus innovative health supplements.
A promising multistage malaria vaccine shows potential for broader protection against the disease, highlighting the evolving role of pharmacists in vaccination efforts.

Discover how AI-engineered nasal antiviral platforms revolutionize flu prevention, enhancing mucosal immunity and offering new treatment possibilities.

The FDA approved the first oral GLP-1 pill for weight management, offering a convenient alternative for obesity treatment and enhancing patient access.
Fam-trastuzumab deruxtecan-nxki (T-DXd) promises enhanced outcomes for HER2-positive early breast cancer patients post-neoadjuvant treatment.

Pharmacists explore new breast cancer therapies and management strategies, enhancing patient care and treatment outcomes at SABCS.
New agreements aim to align US drug prices with European standards, enhancing Medicaid savings and enabling direct consumer purchases.

GLP-1 medications show promise in reducing chemotherapy side effects and improving heart health in breast cancer patients, warranting further research.

Patients learn the importance of understanding treatment-related skin issues, recognizing symptoms, and adopting gentle skincare practices for better management.

Discover how GLP-1 receptor agonists may benefit breast cancer patients by promoting weight loss and improving long-term health outcomes.

FDA approves zoliflodacin, a groundbreaking oral treatment for gonorrhea, offering hope against antibiotic resistance and improving patient access.

Explore the latest insights on breast cancer treatment, focusing on radiation, endocrine therapy, and the role of pharmacists in patient care.