Only 40% of patients with platinum sensitive disease are receiving a PARP inhibitor in the first-line setting.
Alana Hippensteele is lead editor at Pharmacy Times®, Contemporary Clinic, and Pharmacy Times Oncology Edition®. She has a master's in Critical Theory and Creative Research from Pacific Northwest College of Art and a bachelor's degree in English and Art History. She has worked as an English instructor at Temple University and held editorial positions at American Cleaning & Hygiene and Tin House.

Only 40% of patients with platinum sensitive disease are receiving a PARP inhibitor in the first-line setting.
Data from the SOLO-1 trial highlight that even with an excellent prognostic group, patients with ovarian cancer still benefit from the use of a PARP inhibitor.

Black women are at an increased risk of dying from cancer of any type compared to White women, but gynecologic cancers have some of the widest survival gaps.
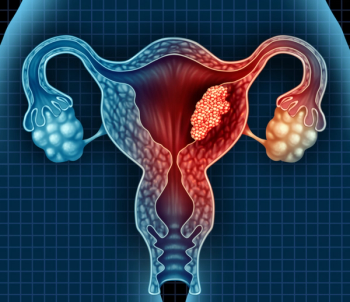
Although poorer prognoses are common for all patients with advanced and recurrent endometrial cancers, minority populations are disproportionately affected with higher rates of mortality.

Several common houseplants present in homes year-round due to their low maintenance requirements and environmental adaptability are far more toxic than many may realize.

The Biden administration announced that it would make 400 million nonsurgical N95 masks for US citizens available free of charge at community health centers and retail pharmacies across the United States.

Study results suggest that infection with Epstein-Barr virus may be the leading cause of multiple sclerosis.

Study data showed that increased levels of infection, inflammation, and metabolic alterations frequently observed in people with depression and psychosis may be a cause rather than a consequence of these disorders.

The emergency use authorization is based on data from the EPIC-HR trial, which showed that the drug significantly reduced the risk of hospitalization or death compared with the placebo.
The drug is available in both oral suspension and tablet dosage forms for use in individuals aged 18 years or younger.

The drug is the first and only oral therapy approved for the treatment of adult patients who are candidates for either phototherapy or systemic therapy.

The agency’s approval follows trial results demonstrating the superior efficacy of the cabotegravir long-acting injectable to a daily oral PrEP option in reducing the risk of infection.

Data support daratumumab as part of a standard of care regimen in the frontline setting for patients with newly diagnosed multiple myeloma.
Patients with relapsed or refractory chronic lymphocytic leukemia are generally divided by those exposed to Bruton's tyrosine kinase inhibitors (BTKi), patients relapsing after venetoclax, and patients relapsing after BTKi and B-cell lymphoma-2 inhibitors.

Alterations in the amino acid profile of children with attention-deficit hyperactivity disorder may support further research into potential new treatment strategies.

The agents that have been evaluated and approved, and those that are coming in the pipeline, make for an exciting time to consider directed therapies.
New treatments show promise for patients with KRAS G12C–mutated disease.

The FDA approved pembrolizumab plus lenvatinib for the first-line treatment of adult patients with advanced RCC.
Erenumab was found to have a sustained efficacy as a monotherapy treatment for episodic migraine that was found to not respond to 2 to 4 prior preventive treatments.

The FDA has approved the monoclonal antibody mepolizumab as a treatment for patients with chronic rhinosinusitis with nasal polyps.

Workflow in the clinic and the pharmacy are critical for the implementation of a new and novel medication therapy, such as cabotegravir-rilpivirine.
Zoster vaccine recombinant, adjuvanted has been approved by the FDA for the prevention of herpes zoster in adults 18 years of age and older who are, or will be, at an increased risk of shingles because of immunodeficiency or immunosuppression.
Trials assessing psychedelic medicine were conducted in the United States for several decades before they were criminalized in 1968, with government and military funding provided for research in the field.
The FDA has approved pembrolizumab in combination with lenvatinib for the treatment of advanced endometrial carcinoma that is not microsatellite instability-high or mismatch repair deficient

The FDA has approved the first and only intravenous immunoglobulin indicated for use in adults with dermatomyositis.

The FDA has approved finerenone for the treatment of adult patients with chronic kidney disease associated with type 2 diabetes.

The FDA has granted enfortumab vedotin-ejfv both a regular approval and a new indication expansion for the treatment of adult patients with locally advanced or metastatic urothelial cancer who are ineligible for cisplatin-containing chemotherapy and have previously received 1 or more prior lines of therapy.
Ribociclib was found to prolong overall survival and improve post-progression outcomes in patients with HR-positive/HER2−negative advanced breast cancer, particularly among younger patients with a significant unmet need.

A study comparing once-monthly injectable galcanezumab-gnlm with rimegepant has been planned to support the assessment of potential treatments to prevent migraines.
Primary results from the randomized portion of the INVICTUS study had previously shown that ripretinib was able to significantly improve progression-free survival with a clinically meaningful overall survival benefit in patients with advanced gastrointestinal stromal tumor.