Article
CDC Recommends Universal Adult Hepatitis B Vaccination: Identification for Pharmacists Just Got Easier
This article was sponsored by Dynavax Technologies.
THE CENTERS FOR DISEASE CONTROL AND PREVENTION (CDC) updated its recommendation on adult hepatitis B vaccination in early 2022 to recommend universal hepatitis B vaccination for all adults aged 19-59 years.1,a
WHY DID THE RECOMMENDATION CHANGE TO UNIVERSAL VACCINATION?
The 1991 universal pediatric recommendation decreased rates of hepatitis B in children; however, many adults born before 1991 are still vulnerable to hepatitis B infections and are advised to be immunized and protected against hepatitis B.2,3 Four out of five adults born before 1991 do not have vaccine-induced immunity.2,3 Health care professionals are critical to the success of the universal adult hepatitis B vaccination recommendation. Identifying patients born before 1991 and delivering a strong recommendation helps protect more patients.1,4,5
Pharmacists and pharmacy technicians are often the first point of contact for patients seeking health care services and typically see a larger number of patients daily compared to in-office health care professionals6; therefore, pharmacists and pharmacy technicians are positioned to provide optimal immunization opportunities. Given their accessibility and frequent patient interactions, pharmacists and pharmacy technicians play a crucial role in expanding access to vaccines and increasing vaccination rates, particularly regarding hepatitis B. With access to patients’ date of birth, pharmacists have the ability to identify eligible patients for immunization opportunities and protect against vaccine-preventable diseases, like hepatitis B. Hepatitis B immunizations are routine vaccinations available throughout the year and recommended for adults aged 19-59 years.1,4,6 Pharmacies are easily accessible and convenient immunization destinations for many patients. Pharmacists and pharmacy technicians can play a critical role in the success of the universal hepatitis B vaccination recommendation.
ROUTINE HEPATITIS B PROTECTION
Utilize these 3 steps to help protect against hepatitis B (FIGURE 1)1,2,5,7,8:
1. Identify adults born before 1991 as patients who may be eligible for vaccination against hepatitis B.1,7
2. Recommend a hepatitis B vaccine by starting a dialogue with your patients that might sound like: “Based on your date of birth, you likely did not receive the hepatitis B vaccine as a child. The CDC recommends that adults get caught up, and I recommend that you get started today. It’s just 2-doses in 1 month and you’re done.”1,2,7,8 A strong recommendation protects more patients.5
3. Protect patients by utilizing HEPLISAV-B [Hepatitis B Vaccine (Recombinant), Adjuvanted], the only 2-dose hepatitis B vaccine for adults aged 18 years and older that is completed with 2 injections over a one-month period.8
HEPLISAV-B
HEPLISAV-B is indicated for the prevention of infection caused by all known subtypes of hepatitis B virus (HBV) in adults aged 18 years and older.8
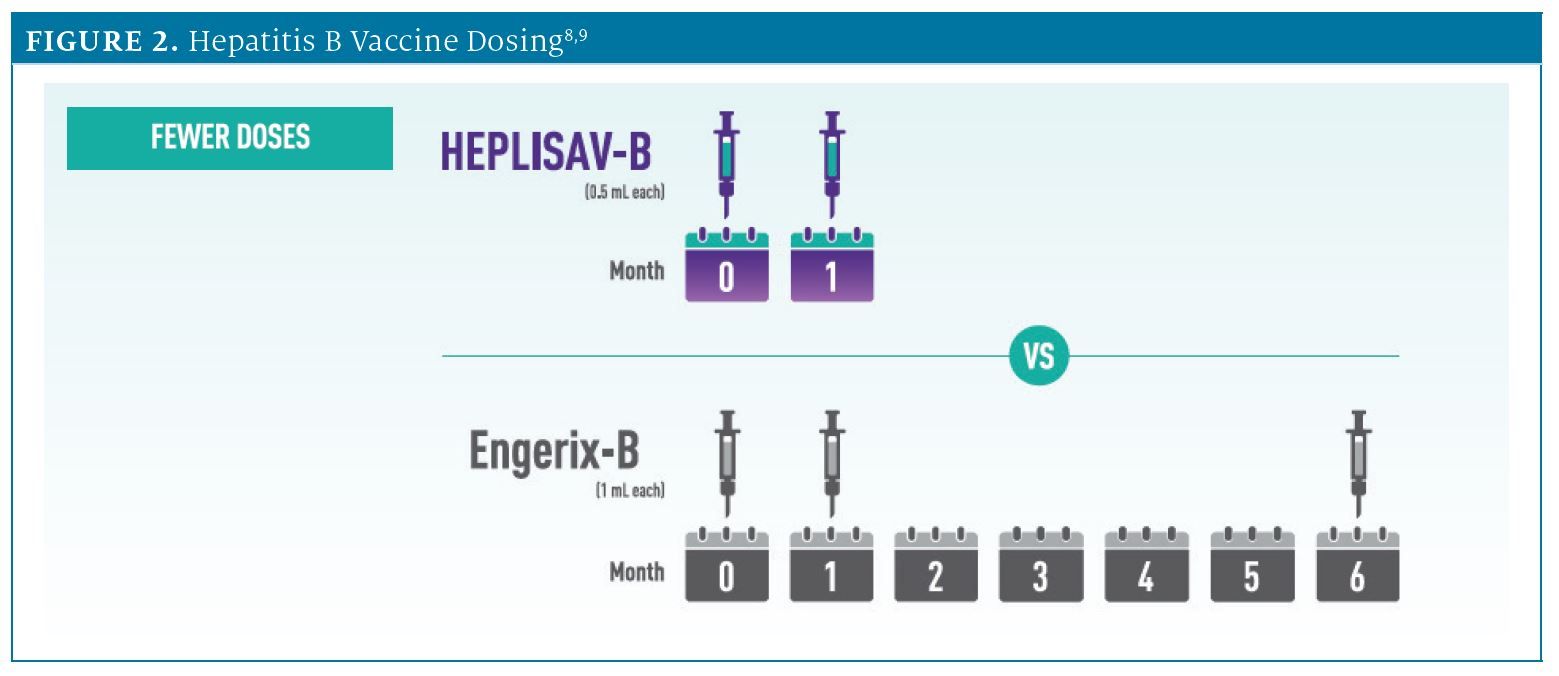
DOSING EFFICIENCY
Series completion is essential for protection with all adult hepatitis B vaccines.8 HEPLISAV-B is the only hepatitis B vaccine completed with 2 doses in 1 month (FIGURE 2).8,9 In a recent survey of 1002 adults, most preferred a 2-dose, 1-month vaccine over a 3-dose, 6-month vaccine.10,b
SAFETY
The safety profile for HEPLISAV-B was demonstrated using data derived from the largest clinical trial safety database for hepatitis B vaccine (N = 14,238).11 The most commonly reported adverse reactions reported within 7 days of vaccination were injection site pain (23%-29%), fatigue (11%-17%), and headache (8%-15%).8
Please see Important Safety Information within this article.
IDENTIFY. RECOMMEND. PROTECT.
Effective universal adult hepatitis B vaccination starts with pharmacists and pharmacy technicians like you.
Identify adults born before 1991 as patients who may be eligible for vaccination against hepatitis B.1,7
Recommend a hepatitis B vaccine by starting a dialogue with patients, as a strong recommendation protects more patients.1,2,5,7,8
Protect patients by educating on the importance of hepatitis B vaccinations and offering to immunize against hepatitis B with HEPLISAV-B [Hepatitis B Vaccine (Recombinant), Adjuvanted], the only 2-dose hepatitis B vaccine for adults aged 18 years and older that is completed with 2 injections over a one-month period.8
For additional information visit: heplisavbhcp.com/pharmacists.
aThe CDC also recommends hepatitis B vaccination for adults aged ≥ 60 years with risk factors. Adults aged ≥ 60 years without risk factors may receive hepatitis B vaccination.1
bIn a survey of 1002 adults in the United States who use a pharmacy and are likely to receive a vaccine at a pharmacy, 71% preferred 2 doses over 1 month, 19% had no preference, and 9% preferred 3 doses over 6 months. Percentages equal 99% due to rounding.10
INDICATION
HEPLISAV-B is indicated for prevention of infection caused by all known subtypes of hepatitis B virus in adults 18 years of age and older.8
IMPORTANT SAFETY INFORMATION
Do not administer HEPLISAV-B to individuals with a history of severe allergic reaction (e.g., anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV-B, including yeast.8
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of HEPLISAV-B.8
Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to HEPLISAV-B.8
Hepatitis B has a long incubation period. HEPLISAV-B may not prevent hepatitis B infection in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.8
The most common patient-reported adverse reactions reported within 7 days of vaccination were injection site pain (23%-39%), fatigue (11%-17%), and headache (8%-17%).8
Please see Full Prescribing Information at heplisavbhcp.com.
REFERENCES
- U.S. Department of Health and Human Services. Recommended Adult Immunization Schedule for ages 19 years or older. Center for Disease Control and Prevention. February 10, 2023. Accessed April 25, 2023.
https://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf - Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19-59 years: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):477-483. doi:10.15585/mmwr.mm7113a1
- He WQ, Guo GN, Li C. The impact of hepatitis B vaccination in the United States, 1999-2018. Hepatology. 2022;75(6):1566- 1578. doi:10.1002/hep.32265
- Murthy N, Wodi AP, Bernstein H, McNally V, Cineas S, Ault K. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older – United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):229-233. doi:10.15585/mmwr.mm7107a1
- Bjork A, Morelli V. Immunization strategies for healthcare practices and providers. In: Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases. 14th ed. Public Health Foundation; 2021:29-42.
- Valliant SN, Burbage SC, Pathak S, Urick BY. Pharmacists as accessible health care providers: quantifying the opportunity. J Manag Care Spec Pharm. 2022;28(1):85-90. doi:10.18553/jmcp.2022.28.1.85
- Immunization Practices Advisory Committee. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep. 1991;40(RR-13):1-19.
- HEPLISAV-B. Prescribing information. Dynavax Technologies Corporation; 2020. Accessed April 26, 2023. https://www.heplisavbhcp.com/pdfs/
Prescribing_Information_HEPLISAV-B_Hepatitis_B_Vaccine_Recombinant_Adjuvanted.pdf - ENGERIX-B. Prescribing information. GlaxoSmithKline; 2021. Accessed April 28,2023. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Engerix-B/pdf/ENGERIX-B.PDF
- Data on file. Dynavax Technologies Corporation; 2022.
- Dynavax Technologies Corporation. FDA Advisory Committee Briefing Document: HEPLISAV-B™ (Hepatitis B Vaccine [Recombinant], Adjuvanted). Presented at: Meeting of the Vaccines and Related Biological Products Advisory Committee; July 28, 2017; Silver Spring, MD.
DYNAVAX and HEPLISAV-B are registered trademarks of Dynavax Technologies Corporation.
© 2023. All rights reserved. US-23-00-00061 June 2023
Newsletter
Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights.

