About the Author
Christie Hurteau is a 2024 PharmD Candidate at the University of Connecticut.
News
Article
Author(s):
Unlike conventional monoclonal antibodies, BsAbs possess dual binding domains that target 2 distinct antigens simultaneously.
Introduction
Multiple myeloma (MM) is a hematologic malignancy characterized by the proliferation of malignant plasma cells within the bone marrow. Multiple myeloma represents approximately 1% of all cancers and 10% of hematologic malignancies.1 In 2024, the American Cancer Society predicts approximately 35,780 new cases of multiple myeloma in the United States and 12,540 estimated deaths. This represents an average lifetime risk of about 1 in 103 men and 1 in 131 women, depending on individual risk factors.1 Treatment goals include minimizing M protein levels, eradication of myeloma cells from the bone marrow, enhancing quality of life with minimal adverse effects, extending the period of response before relapse, and prolonging overall survival.
Image credit: David A. Litman | stock.adobe.com
Standard Therapies
As one of the most common hematologic cancers, MM poses significant therapeutic challenges. Traditional treatment approaches involve a combination of proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies. The oncology team determines whether patients are eligible for chemotherapy and/or autologous stem cell transplant. Standard therapies for MM encompass a range of novel agents and traditional drugs such as proteasome inhibitors, monoclonal antibodies, and immunomodulatory drugs which are listed in Table 1.2 These therapies alone or in combination have significantly improved patient outcomes and survival rates. However, despite advancements in therapy, MM remains incurable, necessitating exploration of novel treatments. Among these, bispecific antibodies (BsAbs) have emerged as promising agents, altering the landscape of MM treatment.
Table 1.2 Multiple Myeloma Therapies

The Emergence of Bispecific Antibodies
Unlike conventional monoclonal antibodies, BsAbs possess dual binding domains that target 2 distinct antigens simultaneously. This unique mechanism enables BsAbs to engage both tumor cells and immune effector cells, enhancing cytotoxicity and tumor cell clearance. Currently, the FDA has approved 3 BsAbs for MM in patients who have undergone at least 4 prior lines of therapy: teclistamab (Tecvayli; Johnson & Johnson), talquetamab (Talvey; Janssen), and elranatamab (Elrexfio; Pfizer).3-5
Adverse Effects and Management of Bispecific Antibodies
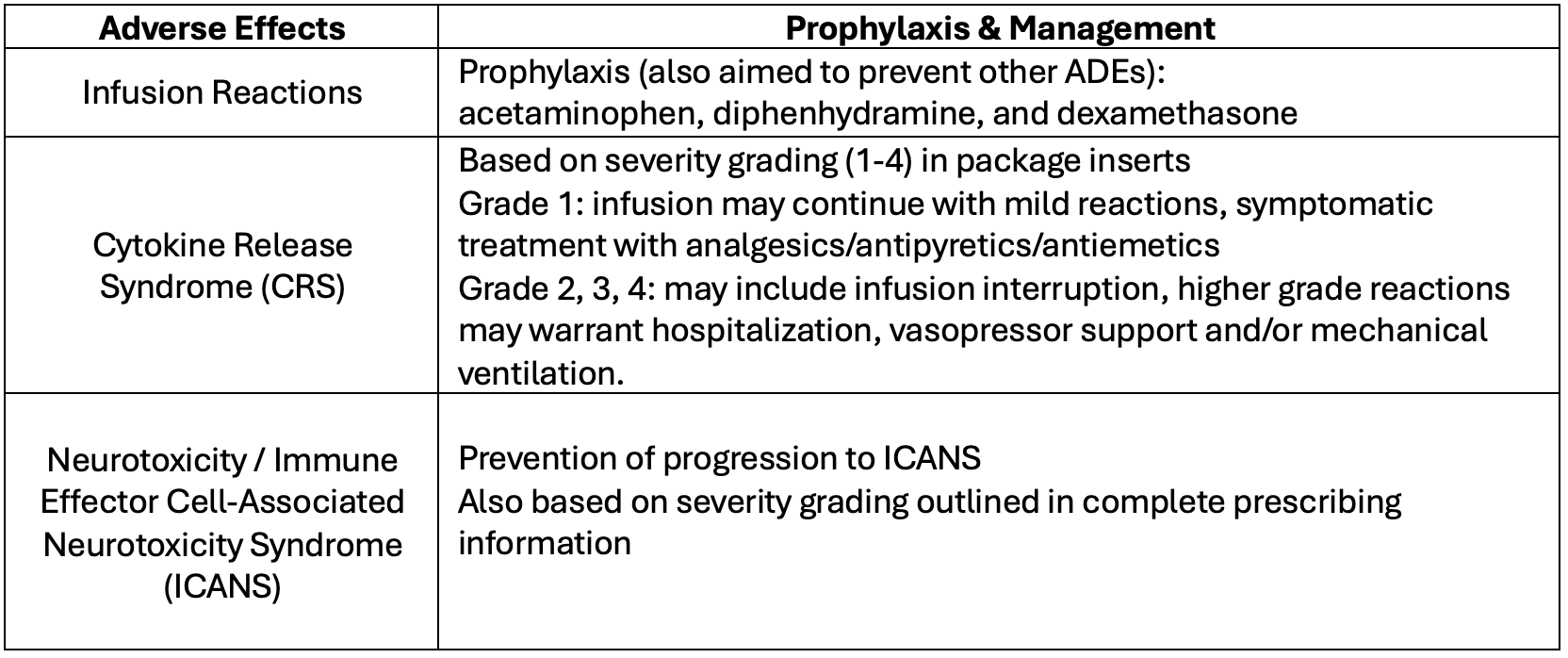
Despite their efficacy, BsAbs are associated with specific known adverse effects, including cytokine release syndrome (CRS) and neurotoxicity that can progress to immune effector cell-associated neurotoxicity syndrome (ICANS). To mitigate these risks, comprehensive management strategies are imperative. BsAbs can only be given through a Risk Evaluation and Mitigation Strategies (REMS) program. Management of CRS and neurologic toxicity is treated based on severity grading. With adequate pretreatment management, the incidences of these toxicities are low. Patients can receive pre-treatment medications to reduce the incidence of CRS and prophylaxis for herpes zoster.6 Table 23-5 lists pre-treatment and management of adverse effects. BsAbs require step-up dosing protocols to mitigate infusion-related reactions and monitoring.
Table 2.3-5 Adverse Effects and Management of Bispecific Antibodies
Step-Up Dosing and Outpatient Protocols
Teclistamab is the first BCMA-targeted bispecific antibody approved for myeloma treatment, administered subcutaneously. It follows a step-up dosing schedule with doses on day 1, day 4, and treatment initiation on day 7, followed by weekly dosing until disease progression.7-10 Premedication given prior to each step-up dose includes steroids, antihistamines, and acetaminophen, with potential hospitalization for 48 hours due to adverse effects.
Inpatient protocols are the currently established standard of care. Outpatient step-up dosing regimens are currently being studied, but there is currently no standardized approach to outpatient dosing, with varied opinions on safety and feasibility.11-13 Caregiver involvement and specific patient criteria are essential for outpatient treatment. Ongoing studies such as the MajesTEC-1 trial (NCT03145181)14 aim to interpret the safety and efficacy of outpatient BsAb administration.
Challenges and Best Practices
Despite promising efficacy, BsAbs are associated with several challenges, including provider and patient hesitancy, logistic barriers, and limited real-world evidence. Addressing these challenges requires collaborative efforts among health care providers, patients, and families to optimize BsAb utilization and improve patient outcomes.
Real-World Evidence and Clinical Trials
Real-world evidence is crucial in supplementing the clinical trial data, providing insight into the landscape of BsAb utilization, safety, and effectiveness in diverse patient populations. The MajesTEC-1 trial demonstrated 61.8% overall response rate (ORR) in patients who received teclistamab.3 Median time to first response was 1.2 months, 90.6% of patients continued to respond at 6 months, and 66.5% of patients continued to respond at 9 months, highlighting its significant efficacy.3 Ongoing clinical trials, including MajesTEC-1, MonumenTAL-1, and MagnetisMM-3, are pivotal in expanding our understanding of BsAbs and their role in MM management.
Conclusion
The introduction of BsAbs represents a significant milestone in multiple myeloma therapeutics, offering a novel approach to target tumor cells while engaging the immune system. Although challenges remain, ongoing research and clinical trials hold promise for further advancing BsAb therapy and improving outcomes for patients with multiple myeloma.
Christie Hurteau is a 2024 PharmD Candidate at the University of Connecticut.
Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights.