
Caroline Carney, MD, MSc, FAPM, CPHQ, chief medical officer of Magellan Health, a board-certified internist, and a board-certified psychiatrist, discusses the correlation between heart health and mental health.

Caroline Carney, MD, MSc, FAPM, CPHQ, chief medical officer of Magellan Health, a board-certified internist, and a board-certified psychiatrist, discusses the correlation between heart health and mental health.

Scott J. Knoer, MS, PharmD, FASHP, EVP and CEO of APhA, discussed the value of the pharmacist on patient care teams and how this value could be supported and enhanced in the future.
Although the CDC and FDA have lifted the pause on Johnson & Johnson’s COVID-19 vaccine, Humphreys said the vaccine now comes with a warning label, and ongoing safety reviews will be paramount to ensure its continued safety.
Kenneth Komorny, PharmD, BCPS, a co-lead on development of National Comprehensive Cancer Network recommendations for biosimilar management, explains some of the positions the NCCN has taken.
Multidisciplinary panel of experts share final thoughts on strategies to normalize naloxone and decrease barriers to its obtainment and utilization.

Charles Argoff, MD, leads a discussion on the use of pain contracts versus pain agreements between clinicians and patients to bring about desired outcomes.

Experts discuss the need for and impact of peer-to-peer discussion initiatives, as well as the impact telemedicine utilization has had on the opioid crisis.
Overcoming barriers for payer coverage of biosimilars as well as considerations for carrying costs are discussed.
Brandon Dyson, PharmD, BCOP, BCPS, shares his approach to switching a patient already on biologic therapy to a biosimilar, and panelists discuss the importance of education regarding the extrapolation of indications for biosimilars.
Historic COVID-19 vaccination efforts have illustrated the value of pharmacists, reinforcing many pharmacy students' desire to be a part of the profession.

Pharmacy Times® interviewed Scott J. Knoer, MS, PharmD, FASHP, the EVP and CEO of APhA, on how the recently introduced Pharmacy and Medically Underserved Area Enhancement Act may impact pharmacy and health care outlooks.
Cynthia Lynch, MD, breast cancer program clinical advisor with Cancer Treatment Centers of America, said oncology pharmacists are vital team members who can help ensure optimal treatment and safety for patients with breast cancer.

For World Immunization Week, Pharmacy Times® interviewed Nurisha Wade, MBA, VP of Global Healthcare Quality & Safety Center of Excellence at US Pharmacopeia (USP), and Farah Towfic, PharmD, MBA, director, CEO of Operations at USP, on USP’s COVID-19 Vaccine Handling Toolkit.

Pharmacy Times® interviewed Scott J. Knoer, MS, PharmD, FASHP, the EVP and CEO of the American Pharmacists Association, on the Pharmacy and Medically Underserved Area Enhancement Act that was recently introduced in Congress.

A discussion on the extrapolation of European data to aid in P&T (Pharmacy and Therapeutics) committee decisions to adopt the use of biosimilars.
Pharmacy Times® interviewed Amy Snyder, a member at Eckert Seamans Cherin & Mellott, on the implications for pharmacy of mandated COVID-19 vaccinations in higher education institutions.

Just as pharmacists have been on the front lines of the COVID-19 pandemic, Kristen Lund, CPhT, a technician at Hy-Vee Pharmacy in Harlan, Iowa, said technicians have taken on greater responsibilities and have become more involved in patient care.

Pharmacy Times® interviewed Dennis O’Neill, president and board member of Biomedican, to discuss how providers are looking to psychedelic medicine to treat certain mental health disorders.

Experts share insights on how they have utilized Prescription Monitoring Programs to combat the opioid crisis and improve the safety of prescribed opioids.

Experts share approaches to normalize conversations about naloxone with patients, as well as share important considerations for maximizing naloxone education.

Experts discuss managing biosimilar adoption in various clinical practice settings, working through the P&T (Pharmacy and Therapeutics) approval process, and the importance of relying on a shared-decision making framework.
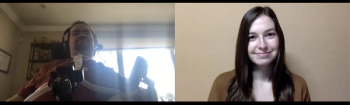
Pharmacy Times spoke with spinal muscular atrophy (SMA) patient Nick Sinagra about his treatment journey and his experiences with 2 different SMA treatments.
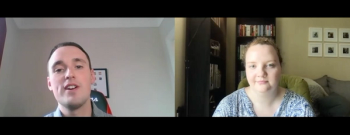
Pharmaceutical companies have already begun developing these boosters, and Haydock said he expects to see some variant-specific vaccines available by mid-2021.

Multidisciplinary panel of clinicians discuss whether recommendations made by national, state, and local agencies infringes upon clinical practice autonomy.
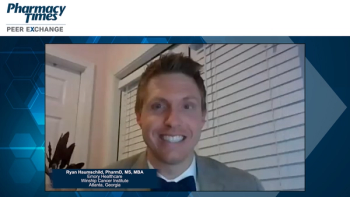
Ryan Haumschild, PharmD, MS, MBA, provides insight on biosimilar adoption both in oncology and non-oncology practice settings.

Jeffrey Bratberg, PharmD, FAPhA, and Joshua Lynch, DO, EMT-P, FAAEM, FACEP, discuss highlights from the December 2020 CDC Health Alert and application.
As the FDA and CDC have paused use of the Johnson & Johnson COVID-19 vaccine in the United States, research is ongoing to understand whether cerebral venous sinus thrombosis (CVST) is directly linked to the vaccine.

The impact on biosimilar utilization in clinical practice caused by secondary patents of biologics and skinny labels of biosimilars is discussed.
Over the past 18 months, the FDA has approved 4 new regimens for HER2-positive metastatic disease.

Madeline Camejo, PharmD, and Estela Trimino, PharmD, BCPS, discuss the emerging role of the pharmacist on the front line of vaccine administration.