
Jordan Tishler, MD, president of the Association of Cannabis Specialists and faculty at Harvard Medical School, discusses his outlook on the future of both care for patients with PTSD and acknowledgement of the role of cannabis in its treatment.

Jordan Tishler, MD, president of the Association of Cannabis Specialists and faculty at Harvard Medical School, discusses his outlook on the future of both care for patients with PTSD and acknowledgement of the role of cannabis in its treatment.

Suzanne Soliman, PharmD, BCMAS, and Sally Arif, PharmD, BCPS, BCCP, discuss how the COVID-19 pandemic has impacted women in the pharmacy field.
Xembify (immune globulin subcutaneous human–klhw) is a 20% immune globulin indicated for treatment of primary humoral immunodeficiency disease in patients 2 years of age and older.
Coordinating complex care plans can involve many care team members and even different pharmacists in both health system and retail environments.

Earlier this year, the FDA granted accelerated approval to tepotinib (Tepmetko, EMD Serono Inc) for the treatment of adults with metastatic non-small cell lung cancer who are harboring MET exon 14 skipping alterations.

Jordan Tishler, MD, president of the Association of Cannabis Specialists and faculty at Harvard Medical School, discusses the importance of unbiased, knowledgeable providers being present at the site of sale of cannabis in order to provide accurate information on the appropriate use of medical cannabis for certain medical conditions.

Susan Lang, MA, MBA, the CEO of XIL Health and former senior executive with Express Grips, discusses what might happen to independent pharmacies if things continue as they are following the financial difficulties of the pandemic.
Susan Lang, MA, MBA, the CEO of XIL Health, discusses how pharmacies that have already been hard hit by the pandemic may be impacted if Pfizer goes through with their projected increased in COVID-19 vaccine prices.

Michael Holden, FRPharmS, FRSPH, associate director of Pharmacy Complete in the United Kingdom, discusses some of the key differences between the role of the pharmacist in the United Kingdom versus the United States.
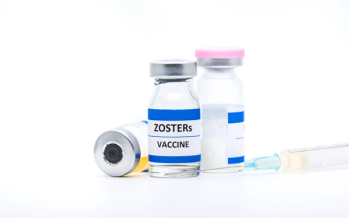
Shingrix is a non-live, recombinant subunit vaccine given intramuscularly in two doses for the prevention of herpes zoster in adults aged 50 years and older.

Because study coordinators were unable to travel, research pharmacist Brian Wortz, PharmD, said researchers were forced to adapt quickly and figure out what they could do remotely.

As the drug experts, Brian Wortz, PharmD, said pharmacists may be able to recognize adverse effects before other health care professionals, ensuring patient safety and optimizing their care.

Oncologist Susan Miesfeldt, MD, of MaineHealth, discussed the importance of genetic testing and counseling for breast and ovarian cancers as well as barriers that must be addressed in order to reach more patients eligible for these services.

Clinical trial participants often have even more questions than patients receiving standard-of-care treatments, so working within the team to answer their questions and provide optimal care is essential for research pharmacists.
Susan Lang, MA, MBA, CEO of XIL Health and former senior executive at Express Scripts, discusses some of the issues pharmacies are experiencing around staffing to support COVID-19 immunizations and how these issues may change post-pandemic.
Dupilumab is a fully human monoclonal antibody that inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) proteins.

To recognize National PTSD Awareness Month in June, Pharmacy Times interviewed Jordan Tishler, MD, on how medical cannabis can be used to treat PTSD among veterans and the general public.

Susan Lang, MA, MBA, CEO of XIL Health and former senior executive at Express Scripts, on some of the ways pharmacies have been affected by the COVID-19 pandemic over the past year.

Because health care is a constantly evolving field, research pharmacist Brian Wortz, PharmD, of Cancer Treatment Centers of America, said pharmacists must be aware of the newest findings and how they could impact their patients.
Pharmacy Times spoke to Dr. Douglas Scharre, neurologist and director of the division of Cognitive Neurology at Ohio State Wexner Medical Center, about the latest FDA approved drug for Alzheimer disease.
Jay Lieberman, MD, a pediatric infectious disease specialist and the senior medical director of PRA Health Sciences, discusses the value of the pharmacist in administering COVID-19 vaccines to adolescents and children.

In an interview with Pharmacy Times, Brian Wortz, PharmD, said he enjoys the innovation in his research, especially when their findings have the potential to drastically impact patients’ lives.
Evinacumab-dgnb is the first FDA-approved treatment that binds to and blocks the function of angiopoietin-like 3, a protein that plays a key role in lipid metabolism.

Working in clinical research combines skills from both the retail and health system pharmacy spaces, as well as other, more unique skillsets, according to research pharmacist Brian Wortz, PharmD, of Cancer Treatment Centers of America, in an interview with Pharmacy Times.
Several third-year pharmacy students at the Washington State University College of Pharmacy and Pharmaceutical Sciences said they were involved with COVID-19 testing and vaccination efforts, including organizing mass vaccination events and testing patients in their communities.

Jonathan Spicer, MD, medical director of the McGill University Health Center Thoracic Oncology Network, discusses what is still needed to be investigated in the future following the phase 3 trial assessing nivolumab plus platinum-doublet chemotherapy as neoadjuvant treatment for resectable non-small cell lung cancer.

Jonathan Spicer, MD, medical director of the McGill University Health Center Thoracic Oncology Network, discusses what some of the most important findings were in regard to patient outcomes from the phase 3 trial assessing nivolumab plus platinum-doublet chemotherapy for resectable non-small cell lung cancer.

Research pharmacist Brian Wortz, PharmD, with Cancer Treatment Centers of America, said working as a pharmacist in clinical research provides interesting opportunities to be at the forefront of drug development.
Jonathan Spicer, MD, medical director of the McGill University Health Center Thoracic Oncology Network, discusses findings regarding adverse events during the phase 3 trial assessing nivolumab plus platinum-doublet chemotherapy as neoadjuvant treatment for resectable non-small cell lung cancer.

Jonathan Spicer, MD, medical director of the McGill University Health Center Thoracic Oncology Network, discusses what the findings of the phase 3 CheckMate 816 trial assessing nivolumab plus platinum-doublet chemotherapy as neoadjuvant treatment for patients with resectable non-small cell lung cancer may mean for future treatment options.