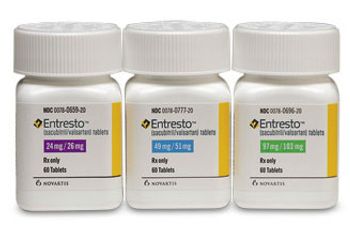
ENTRESTOâ„¢ (sacubitril/valsartan) tablets 24/26 mg, 49/51 mg, 97/103 mg, from Novartis Pharmaceuticals Corporation, are a combination of an angiotensin receptor blocker and a novel neprilysin inhibitor, indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (New York Heart Association class II through IV) and reduced ejection fraction.