
Rachel Rubin, MD, an assistant clinical professor in urology at Georgetown and a urologic surgeon, discusses hypoactive sexual desire disorder and the current treatments available for this medical condition.

Rachel Rubin, MD, an assistant clinical professor in urology at Georgetown and a urologic surgeon, discusses hypoactive sexual desire disorder and the current treatments available for this medical condition.

Ronald T. Piervincenzi, CEO of US Pharmacopeia, discusses how building resilience in the medicine supply chain will help bolster preparedness for what may arise during the COVID-19 pandemic and beyond.

This Valentine’s Day, pharmacists can remind patients that they may not need to feel guilty when they indulge in a small piece of chocolate.

Envive reduces bloating, diarrhea, constipation, gas, and discomfort.

Tresiba is indicated to improve glycemic control in patients with diabetes.

Bebtelovimab gets Emergency Use Authorization to treat mild-to-moderate COVID-19 in adults and pediatric patients who are at high risk for progression to severe COVID-19.

Ronald T. Piervincenzi, CEO of US Pharmacopeia, discusses contributing factors leading to health care professionals experience of an erosion of trust in the medicine supply chain.
Tiago Reis Marques, MD, PhD, CEO of Pasithea Therapeutics, discusses the future of ketamine and psychedelic medicines in the treatment of mental health and brain disorders.

In the fourth episode of Coffee With Suzy, we listened back to some of the best moments from the series so far.
The drug's novel molecular structure may result in fewer toxic adverse effects.
New analysis shows the substitution would cut costs by $600 million in the United States annually.
American Heart Month is another example of the essential and valuable position that pharmacists hold in improving health care outcomes and optimizing patient-centered care.

Ronald T. Piervincenzi, CEO of US Pharmacopeia, discusses how drug shortages are related to medicine quality issues.
Study shows that 98% of cases of multi-system inflammatory syndrome in children (MIS-C) were unvaccinated, suggesting that vaccination reduces the chance of a child who had COVID-19 later developing MIS-C.

Genentech has released new 2-year data for individuals with diabetic macular edema and wet age-related macular degeneration.

The lingering effects of the COVID-19 pandemic, including supply concerns and staffing shortages, have made technological solutions become more crucial.
Ronald T. Piervincenzi, CEO of US Pharmacopeia (USP), discusses recent survey data demonstrating 90% of physicians had experienced an erosion of trust in the US supply chain’s ability to deliver safe, quality medicines.

Joint Complex supports joint function and lubrication, as well as tissue hydration.
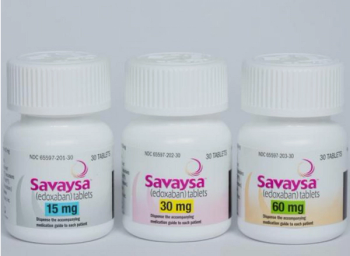
Edoxaban (Savaysa) can reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.

Sexual health is directly linked to mental health, Ramsden said, and both issues often go under-investigated in women.

Tiago Reis Marques, MD, PhD, CEO of Pasithea Therapeutics, addresses how pharmacists can respond to questions patients may ask them about ketamine and other psychedelic treatments.

Tiago Reis Marques, MD, PhD, CEO of Pasithea Therapeutics, explains how ketamine infusions work when administered in patients’ homes and what safety precautions would be taken by providers in this setting.

A variety of drugs from several medication classes are under investigation.

New composite pharmacy adherence measure is a major step in developing standard pharmacy performance measures focused on improving patient care.

Increasing physical activities and reducing caloric intake isn’t always enough to conquer obesity.

Many faculty members are adept at training and teaching pharmacists and pharmacy technicians how to perform physical exams and tests.
Taking appropriate doses of vitamins and minerals may aid in lowering the risk of cardiovascular disease.

Study shows that treatment with rituximab (Rituxan) for B-cell non-Hodgkin lymphoma prior to COVID-19 vaccination significantly lowered the number of patients who developed blocking antibodies for the virus.

Lack of immediate results, cultural barriers, and high costs lead many to gamble with their cardiovascular health, study results show.

An emerging focus is aligning targeted, safe, and effective therapies with patient goals.