Under the proposed plan, Medicare payments for Part B drugs would be based on international prices.

Under the proposed plan, Medicare payments for Part B drugs would be based on international prices.

Top news of the day from across the health care landscape.

Top news of the day from across the health care landscape.

Three hospitals made significant progress in reducing deaths associated with poor quality health care.

Study evaluates when and how the increased risk of autoimmune thyroid disease develops in patients diagnosed with rheumatoid arthritis.

Bijuva (estradiol and progesterone) is the first and only FDA-approved bio-identical hormone therapy combination of estradiol and progesterone in a single, oral capsule for the treatment of moderate to severe vasomotor symptoms due to menopause in women with a uterus.

According to TherapeuticsMD, Bijuva is the first and only FDA-approved bio-identical hormone therapy combination of estradiol and progesterone in a single, oral capsule for the treatment of moderate to severe vasomotor symptoms due to menopause in women with a uterus.

Despite a decline in the rate of opioid prescribing, the CDC reports that the number of opioid related deaths continues to rise. The pharmacology and pharmacokinetics of prescription and illicit fentanyl could help to shed light on why the opioid epidemic has taken the path that it has.
Sun Pharma announced the availability of its treatment for moderate-to-severe plaque psoriasis in the United States.

Biosimilars adalimumab and etanercept showed equivalent safety and efficacy to reference products in patients with rheumatoid arthritis.

New data show a survival benefit with pembrolizumab (Keytruda) as both a monotherapy and combination treatment with chemotherapy.

Top news of the day from across the health care landscape.

Top news of the day from across the health care landscape.

Officials with the FDA have approved Jazz Pharmaceuticals' Xyrem (sodium oxybate) for the treatment of cataplexy and excessive daytime sleepiness in pediatric patients aged 7 to 17 years with narcolepsy.

Hepatitis C virus found to increase mortality in individuals coinfected with HIV, however, treatment with direct-acting antivirals may reduce harm.
Costume contact lenses are gaining popularity, but there are concerns with these products.

Having a pharmacy that truly matters can make all the difference in the world to your patients, and to your bank account.

In the lexicon of management, the owner is the epitome of leadership. Yet surprisingly, according to the Harvard Business Review, little is known about this unique role.

Officials with the FDA have approved Jazz Pharmaceuticals' Xyrem (sodium oxybate) for the treatment of cataplexy and excessive daytime sleepiness (EDS) in pediatric patients aged 7 to 17 years with narcolepsy.

In this series, Jill Drury, PharmD, a clinical pharmacy specialist, provides patients with "Brown Bag" consultations. Patients bring to the pharmacy their current medications and over-the-counter products, giving the pharmacist an additional resource for pharmaceutical counsel. In this video, a patient with fair skin is planning a tropical vacation. She seeks advice about topical skin protection and potential adverse effects from the sun with her medications.

Several studies represent some of the important clinical trials in the treatment of Non-Hodgkin's Lymphoma.
Tildrakizumab-asmn (Ilumya) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults.

Top news of the day from across the health care landscape.

Top news of the day from across the health care landscape.
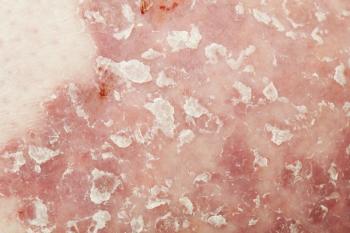
Tildrakizumab-asmn (Ilumya), an injectable interleukin-23 (IL-23) inhibitor, was approved by the FDA in March for adults who are candidates for systemic therapy or phototherapy.

Top news of the week from Specialty Pharmacy Times.
Phase 3 data show combination therapy demonstrates clinical benefit as first-line treatment.

A antiviral has been FDA-approved, just in time for flu season.

Amgen has announced that it will reduce the price of its cholesterol drug evolocumab (Repatha) by 60%, from its annual list price of $14,100.
Amgen will make evolocumab (Repatha) available at a list price of $5850, a 60% reduction from its annual list price of $14,1000.