
Ruxolitinib is the first and only FDA-approved treatment for this indication, according to Incyte, the drug's manufacturer.

Ruxolitinib is the first and only FDA-approved treatment for this indication, according to Incyte, the drug's manufacturer.

Officials with the FDA have approved alpelisib (Piqray, Novartis) tablets, to be used in combination with the endocrine therapy fulvestrant, to treat postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer following progression on or after an endocrine-based regimen.

The drug, onasemnogene abeparvovec-xioi (Zolgensma, AveXis), is an adeno-associated virus vector-based gene therapy that targets the cause of SMA, a leading genetic cause of infant mortality.

The drug, onasemnogene abeparvovec-xioi (Zolgensma, AveXis), is an adeno-associated virus vector-based gene therapy that targets the cause of SMA, a leading genetic cause of infant mortality.

The study included an analysis detailing crash and traffic violation records for newly licensed drivers. It is the first large-scale longitudinal study on this topic.

Top news of the week from Specialty Pharmacy Times.

Researchers seek to investigate how to broaden the potential of cediranib with and olaparib to treat multiple cancer types.

Top news of the day from across the health care landscape.
Understanding what constitutes a 'good' immune response to HIV may provide important information for vaccine design and intervention.
Among the most prevalent symptoms of chimeric antigen receptor T-cell therapy is encephalopathy.
Study suggests a possible co-treatment strategy to overcome resistance to Venetoclax in the treatment of chronic lymphocyte leukemia and acute myeloid leukemia.
NKTR-181 is currently under review with the FDA, with a Prescription Drug User Fee Act (PDUFA) target action expected in August 2019.

Top news of the day from across the health care landscape.

According to the ADA, best practice is to pack at least twice as much medications and glucose testing supplies as normally needed to minimize complications due to travel delays or lost luggage.3 Many manufacturers of insulin pumps will provide a loaner for international travel.

Calcipotriene foam was approved by the FDA in 2010 for patients age 18 years and older.

Regulatory affairs is another frontier for pharmacists to explore, and pharmacists are just scratching the surface of the opportunities in this area. In this interview with pharmacist Brendan Doran of CTI Clinical Trial & Consulting, he touches on the many phases of the drug life cycle with an emphasis on the process of bringing a drug to regulatory bodies for market approval, and gives a look into some of the current and emerging roles available in regulatory affairs.

Results of study demonstrate safety profile in pediatric patients.

The number of cases of the disease in 2016 was the highest since 1972 and represents a decades-long trend.
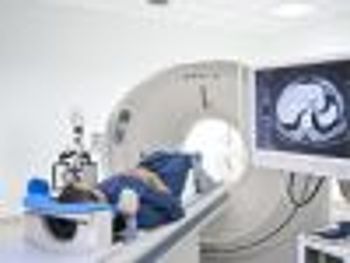
Using real-time data helped providers identify racial disparities in cancer treatment and narrow the gap in treatment completion rates between white and black patients.
Study examines cutting off glutamine and fatty acids, two well-known cancer food sources that tumors use to grow and survive.

Top news of the day from across the health care landscape.

This ad was supported by Church & Dwight Co. Inc., featuring Feryal Hajee, MD.
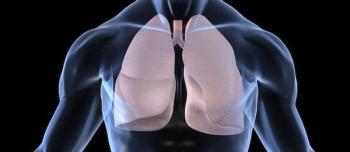
The new phase 2 data for an investigational once-daily, fixed dose combination currently in development for the treatment of inadequately controlled asthma was presented at the 2019 Annual International Congress of the American Thoracic Society (ATS) in Dallas.

The acquisition agreement with Peloton includes the drug candidate PT2977, a novel oral HIF-2α inhibitor in late-stage development for renal cell carcinoma (RCC).

Solifenacin succinate tablet is a muscarinic antagonist indicated to treat this condition, with symptoms of urgency, urge urinary incontinence, and urinary frequency.

Research may lead to potential new targets for preventing cancer in humans.

Glenmark Pharmaceuticals and Alembic Pharmaceuticals have both received FDA approval for generic Solifenacin Succinate tablets.

Statins can benefit patients with secondary progressive multiple sclerosis for reasons unrelated to their cholesterol-lowering effects.

Top news of the day from across the health care landscape.

Midazolam (Nayzilam) is indicated for seizures that are distinct from a patient's usual seizure pattern, in individuals age 12 years and older with epilepsy.