
Demcizumab is a DLL4-targeted lgG2 humanized monoclonal antibody and a potent inhibitor of the Notch pathway.

Demcizumab is a DLL4-targeted lgG2 humanized monoclonal antibody and a potent inhibitor of the Notch pathway.
COA released a statement explaining that it fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs due to concerns regarding the safety of patients in such an environment.

Derek Angus, MD, MPH and his collaborators had developed a platform called REMAP-Community Acquired Pneumonia (REMAP-CAP), which was designed to find optimal treatment for severe pneumonia both in non-pandemic and pandemic settings.

Every pharmacy within the Guardian Pharmacy Service network has their own disaster contingency plan in place so that they are supported in an emergency, such as the coronavirus disease 2019 pandemic.
Top news of the week from Pharmacy Times surrounding the novel coronavirus.

Today we’re celebrating the work of Andrea Torres, CPhT, who works at Axtell Pharmacy in Pilot Point, Texas.
A case study of a patient in Wuhan, China suggests that the immunosuppressant tocilizumab may be an effective coronavirus disease 2019 treatment for patients with multiple myeloma and other blood cancers.
By mining a vast trove of genetic data, researchers are enhancing physicians’ ability to treat cancer, predict patient outcomes, and determine which treatment will work best for individual patients.
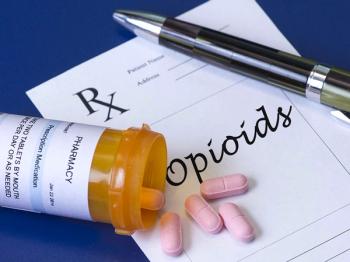
Research explores the impact of obesity on prescription opioid use, investigating specifically the link between obesity and long-term prescription use.

Research from Wuhan, China, found that those with diabetes and high blood pressure were overrepresented among severely ill patients and those who have passed away from COVID-19.
The investigators from the University of Michigan emphasized that COVID-19 may not necessarily behave like other coronaviruses and influenza outbreaks.

Which ancient pharmacist created procedures that still influence today’s modern compounding practices?
FDA updates guidelines after man who recently recovered from COVID-19 was told that he could not donate blood for 12 months because he takes Truvada as a pre-exposure prophylaxis treatment.

This medication inhaler is indicated for the treatment or prevention of bronchospasm in patients ages 4 years and older who have reversible obstructive airway disease.

The antiviral drug remdesivir was originally developed as a treatment for Ebola in 2014, but upon the surge of COVID-19, its use was investigated in treating COVID-19.

Michael Klepser, PharmD, FCCP, FIDP is not only a part of this group, but was also a potential carrier of the virus himself.

In response to the financial crisis driven by the coronavirus disease 2019 (COVID-19) pandemic, drug manufacturers are aiming to increase access to insulin products.

More than one-third of transgender women (TGW) not living with HIV at baseline were indicated for pre-exposure prophylaxis (PrEP).

US Department of Health and Human Services (HHS) Secretary Alex Azar released a statement on how the authorization for pharmacists will mean easier access for patients who need COVID-19 testing.

Today we’re celebrating Lendi Muehlbauer, RPh, who works at a Rite Aid in New Jersey.

The FDA has approved encorafenib in combination with cetuximab for the treatment of adult patients with metastatic colorectal cancer with a BRAF V600E mutation after prior therapy.

With all the factors of the COVID-19 outbreak taken into consideration, simply maintaining your current body weight and level of health and fitness is a win worth celebrating.

Consuming a diet high in fiber was linked with a reduced incidence of breast cancer in an evaluation of all relevant studies, according to findings published in CANCER.

Owen Mumford, Inc is working with GEMA to donate 500,000 Unistik safety lancets and 5,000 Peezy urine specimen collection kits.

Access to OmniSYS Training Center supports pharmacists as front-line responders.

Coronaviruses are known to utilize the ACE2 to help facilitate entry into the host target cell.

In this episode of OnScript with NHA, Jeremy Sasser and Jessica Langley talk with Rick Richey, master instructor for the National Academy of Sports Medicine.

ASHP’s 2020 Residency Match concluded with 5269 individuals matching with 2551 pharmacy residency programs across the country.

Understanding patients' perspectives help engender their trust and loyalty, and affect positively their treatment outcomes and the pharmacy business.
PittCoVacc produces antibodies specific to SARS-CoV-2 at quantities thought to be sufficient for neutralizing COVID-19 within 2 weeks.