Research may address the lack of effective treatments for mental fatigue among patients with multiple sclerosis.

Research may address the lack of effective treatments for mental fatigue among patients with multiple sclerosis.

Furthermore, the investigators noted that choline levels high enough to protect the fetus often require dietary supplementation, which offers a chance for pharmacists to provide counseling.

Contrary to popular belief, a new study suggests that music training does not improve academic performance.

This year’s annual NASP meeting will feature an enhanced virtual environment that allows attendees to earn required CPE credits, share best practices, and browse a convention hall.
The American Cancer Society is renewing efforts to increase HPV immunization as COVID-19 threatens to slow cancer prevention progress.
National Association of Chain Drug Stores (NACDS) President and CEO Steven C. Anderson issued a statement on July 31 regarding the nation’s preparations for the eventual deployment of COVID-19 vaccines.

Home infusion of teprotumumab-trbw (Tepezza), a breakthrough treatment for thyroid eye disease, was found to effectively minimize the chance of infection with coronavirus disease 2019 for both patients and staff of Chartwell Pennsylvania, LP.

The investigators noted that the shift toward value-based care has placed a greater emphasis on preventive care and chronic disease management services as delivered by multidisciplinary health care teams.

In the United States, these findings would translate to more than 269,000 prevented breast cancer cases and nearly 44,000 prevented cases of ovarian cancer.

Pharmacists offer a variety of services that can help patients to manage diabetes and other chronic conditions, especially during the COVID-19 pandemic.

Vitamin K levels show observational relationship with risk of death in older population.
A new treatment that targets the physical properties of the herpes virus, not just the virus proteins, shows promise.

What is causing the good changes in this man’s blood work?

The approval represents the first for a second-line treatment for adult patients who progressed during or after first-line therapy.

The American Society of Health-System Pharmacists (ASHP) has announced that the 2020 Midyear Meeting, scheduled to be held December 6-10, 2020, will be entirely virtual.

There is no perfect moment to timestamp the accelerated, massive movement toward finding and distributing the first vaccine candidates for the coronavirus 2019 pandemic.

Representatives from the organizations spoke with HHS last week before President Donald Trump signed several executive orders, including one that would address manufacturer rebates also collected by PBMs.
According to researchers at the Asan Medical Center in Seoul, South Korea, patients who have left their shingles symptoms untreated have an increased risk of dementia.
According to a study published in Genome Medicine, the disruption of gut bacteria through the use of antibiotics soon after birth has the ability to affect the immune system’s process of maturation.

What was the first federal law that required drugs to be proven safe prior to use?

Top news of the week from Pharmacy Times®.

Officials with the FDA have approved atezolizumab plus cobimetinib and vemurafenib for the treatment of patients with BRAF V600 mutation-positive advanced melanoma.

A goal of both projects is to stimulate and demonstrate the impact of optimizing health information technology that enhances medication safety, and to improve patient outcomes.

Pharmacists can play an important role in substance abuse awareness and prevention, and the series is funded by DisposeRx.

Pharmacists, researchers, and other health care professionals must keep pushing these boundaries to continue advancing the field of medicine and providing effective patient care.
The study emphasized the population below the poverty line, for whom food insecurities can make vitamin and mineral intake even more challenging.

Lilly is helping people living with diabetes manage the cost of insulin with affordability solutions.
In a recent study, researchers found that Zoster Vaccine Live, a type of shingles vaccine, may prevent stroke in some older adults.
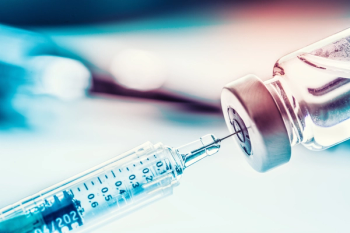
A virtual event was hosted on July 28 by the Economic Club of Washington, DC, on how to immunize Americans as fast as possible upon the release of a COVID-19 vaccine.

Practicing according to HIPAA standards, though, is not just to protect yourself legally, but it is the right thing to do by way of patient care, particularly through the ethical tenets of beneficence and patient autonomy.