Patients at risk for diabetes should try to focus on adhering to their medications, diet and exercise to reduce their blood sugars, which will decrease their A1C levels.

Patients at risk for diabetes should try to focus on adhering to their medications, diet and exercise to reduce their blood sugars, which will decrease their A1C levels.
The investigators also predicted that the increase in indications of novel diabetes treatments and their increased use is likely to result in greater expense.

In a study, median OS times for patients reporting such fatigue were approximately 26% to 45% shorter than those for patients without baseline fatigue.

Pharmacy Times interviewed Craig Freyer, PharmD, BCOP, and Andrew Lin, PharmD, BCOP, to discuss how to manage the logistical challenges that arise during the administration of CAR T therapy in inpatient and outpatient settings for patients with multiple myeloma.

The analysis used AstraZeneca’s global safety database, which contains all spontaneously reported adverse events from real-world use of its medicines and vaccines worldwide.

Anifrolumab is a first-in-class type I interferon receptor antibody and is the only new treatment in more than a decade for this patient population.

The gluten-like proteins found in ryegrass could be interacting with crops commonly used as wheat replacements, which may cause a reaction among people with celiac or gluten intolerance.

John M. O’Brien, PharmD, MPH, newly appointed President and CEO of the National Pharmaceutical Council, discusses his plans for the future of the organization.

In addition to getting outside and taking time to enjoy hobbies outside of work, Carroll urged pharmacists to enjoy time with family and friends whenever possible.

Complications of varicella-zoster virus in patients with lupus may be attributable to immunological abnormalities, lymphopenia conditions, and immunosuppressive therapies.

You read that correctly; drop the zero in 20 to explain spending in the sector in the near future.

The American Cancer Society estimates that oncologists will diagnose about 34,920 new cases of multiple myeloma in 2021 with about 12,410 deaths expected to occur.

This week on Pharmacy Times, there are a number of important topics that will be covered and posted throughout the week.
The investigators noted that the levels of islatravir in peripheral blood mononuclear cells remained above the efficacy pharmacokinetics threshold for PrEP for both doses studied (60 mg and 120 mg) for 8 weeks following the last study dose.
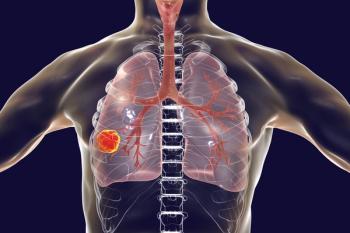
As treatments for lung cancer move forward and patient care services continue to expand, the role of the pharmacist in oncology is critical.

Vviloxazine (Qelbree) is a norepinephrine reuptake inhibitor that provides a new treatment option for patients aged 6 to 17 years with ADHD.
Reducing blood pressure is the major determinant of reduction in cardiovascular risk in both young and older patients with hypertension.

Selexipag tablets were first approved by the FDA in 2015 to delay disease progression and reduce the risk of hospitalization for PAH.

Penny pinchers, early arrivals, and more are featured in this month's Pet Peeves.


The FDA has approved the monoclonal antibody mepolizumab as a treatment for patients with chronic rhinosinusitis with nasal polyps.

Sarah Cannon and Pontchartrain Cancer Center will join the esteemed program.

The Pharmacy Times® Pharmacy Focus podcast provides the latest industry news and information, thought-leader insights, clinical updates, patient counseling tools, and innovative solutions for the everyday practice and business of pharmacy.

The study demonstrated robust time and dose-dependent lowering of tau protein in cerebrospinal fluid (CSF) over the course of the 3-month treatment period.

Investigators estimated that globally, 4.1% of all new cancer diagnoses in 2020 were attributable to alcohol consumption.

This month's products include a smart pen cap for insulin dosing, a treatment for benign prostatic hyperplasia, and more.
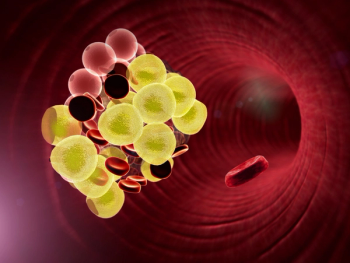
Lipophilic statins for dyslipidemia penetrate muscle more easily than hydrophilic statins and are associated with a higher incidence of adverse effects.

On average, those who received a flu-like diagnosis changed their cell phone usage behavior 1 day before their diagnosis and the 2 to 4 days afterward.

The availability of insulin biosimilars can bring high-quality, lower cost treatment options for pharmacists, physicians, payers, and patients alike.