
The drug’s application was designated for fast track and priority review, as well as orphan product status.

The drug’s application was designated for fast track and priority review, as well as orphan product status.

Rituximab-abbs (Truxima, Celltrion) is the fifteenth biosimilar approved by the FDA and the sixth biosimilar approved in 2018, thus far.

Rituximab-abbs (Truxima, Celltrion) is the fifteenth biosimilar approved by the FDA and the sixth biosimilar approved in 2018 thus far.

CVS Health announced that it has closed its acquisition of Aetna with the promise to transform the consumer health experience.

CVS Health announced that it has finalized its acquisition of Aetna with the promise to transform the consumer health experience.

CVS Health announced that it has finalized its acquisition of Aetna, representing another example of the shift toward major vertical consolidation in the health care industry.

Both actions follow similar recalls of valsartan-containing products by other manufacturers.

Top news of the day from across the health care landscape.

Amazon’s entry into the pharmacy space could be the start of significant changes in the specialty space.

Top news of the day from across the health care landscape.
Teva Pharmaceuticals has initiated a voluntary recall in the United States, to the patient level, of all lots of Amlodipine/Valsartan combination tablets and Amlodipine Valsartan/Hydrochlorothiazide combination tablets, due to an impurity detected above specification limits in an active pharmaceutical ingredient (API) manufactured by Mylan India.
The Task Force notes in the draft recommendations that clinicians should screen everyone between the ages 15 to 65 years and all pregnant women for HIV.

Fostemsavir (ViiV Healthcare) may be a potential new option for patients with HIV who have become resistant to other medications.

Top news of the day from across the health care landscape.

Top news of the day from across the health care landscape.
A new study indicates that there are age-associated gender differences seen in the disease burden of chronic obstructive pulmonary disorder (COPD), with younger women more severely affected.

New recommendations urge health care providers to offer pre-exposure prophylaxis (PrEP) to individuals at high risk of HIV.

Purdue University researchers have developed a shoe insole that could help make the healing process more portable for the 15% of Americans who develop ulcers as a result of diabetes.

The FDA has granted an accelerated approval to larotrectinib (Vitrakvi, Loxo Oncology) for the treatment of adult and pediatric patients with solid tumors that have an NTRKgene fusion without a known acquired resistance mutation, are metastatic or where surgical resection is likely to result in severe morbidity, and have no satisfactory alternative treatments or that have progressed following treatment.

The FDA has granted an accelerated approval to larotrectinib for the treatment of adult and pediatric patients with solid tumors that have an NTRK gene fusion.

Top news of the day from across the health care landscape.

Officials with the FDA have approved Genentech’s single-dose, prefilled autoinjector ACTPen 162 mg/0.9 mL for tocilizumab (Actemra) for patients with rheumatoid arthritis (RA), giant cell arteritis (GCA), and 2 forms of juvenile arthritis.

The single-dose, prefilled autoinjector for tocilizumab (Actemra) offers an additional option for patients with rheumatoid arthritis, giant cell arteritis, and 2 forms of juvenile arthritis.

Top news of the day from across the health care landscape.
Myriad reasons may prevent patients from accessing specialty drugs, which create significant problems for patients in need of treatment.

Antiretroviral drug treatment was not found to be a cause of the higher prevalence of hypertension in persons infected with HIV.

FDA officials cited the possibility of a cardiovascular (CV) safety risk based on Zafgen's prior compound and outlined potential paths for moving forward.

More than 160 patients have thus far been treated with investigational therapeutics under an ethical framework developed by WHO.

Can you solve the pharmaceutical mystery? Each week, a new case study is presented.
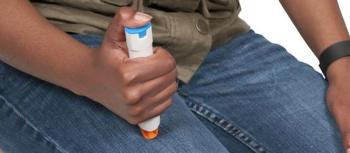
The single-dose, prefilled autoinjector for tocilizumab (Actemra) offers an additional option for patients with rheumatoid arthritis, giant cell arteritis, and 2 forms of juvenile arthritis.