
On the heels of Women Pharmacist Day on October 12, we highlight 3 female pharmacists who have followed unique career paths that help to drive innovation and change in the industry, beyond the retail pharmacy setting.

On the heels of Women Pharmacist Day on October 12, we highlight 3 female pharmacists who have followed unique career paths that help to drive innovation and change in the industry, beyond the retail pharmacy setting.
The diagnostic test is a companion to vorasidenib, an isocitrate dehydrogenase inhibitor that received FDA approval this past summer.

Digital eye strain is a common cause of dry, red, and itchy eyes.

Non–FDA-approved medications may be accessed for patient care via 3 alternative pathways: expanded access, the Right to Try Act, and off-label use, which are reviewed in this article.

A dedicated community pharmacist for over 40 years, Kessler has witnessed immense change and innovation throughout his career.
The story of pinksocks and the inspiring movement that they launched.

In a study, RK-33 demonstrated positive effects against the protein DDX3, which is key in the growth of cancer cells and spreading of disease.
The connection of F proteins could help destabilize the virus prior to infection of the next host.
Zolbetuximab is the first approved CLDN18.2-targeted treatment for gastric and gastroesophageal junction adenocarcinoma.
Increasing serum vitamin B12 before and after the onset of pancreatitis reduced the severity of the condition.
The results emphasize the need for pharmacists and health care providers to further educate patients about receiving recommended immunizations.
The additions include Dextrose 70% intravenous (IV) solution, Lactated Ringers IV Solution, and Peritoneal Dialysis Solution, which were affected by Hurricane Helene.

Recognizing repeat dosing efforts is important to improving outcomes in patients with Guillain-Barré syndrome who do not respond to IVIG.
The tool can detect cell changes and mutations that drive resistance and relapse.
Disruptions in the salience network function are shown to influence the severity of mild behavioral impairment (MBI) symptoms.
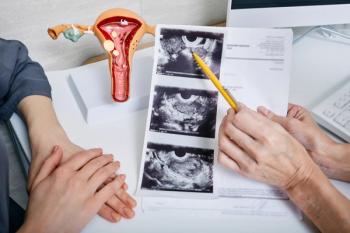
Awareness of uterine fibroids and uterine fibroid embolization can prevent unnecessary hysterectomies, especially in Black women, who are more likely to have uterine fibroids.

Pharmacists today are an integral part of a patient’s care journey, providing clinical expertise and support that improves medication adherence, increases access to medication and advances health outcomes.

Research funding that aims to understand impacts of systemic racism on pediatric asthma should include plans to undo policies that maintain inequities created by systemic racism.
This review discusses the significance of the FDA-approved drug inotuzumab ozogamicin in pediatric patients with acute lymphocytic leukemia (ALL) and relapsed/refractory (R/R) ALL, and the evolving therapeutic options in R/R ALL.

The clinical hold is a result of a reported case of motor neuropathy in a phase 2 trial participant.
Compared with brentuximab vedotin and chemotherapy, nivolumab and chemotherapy had longer progression-free survival and a better safety profile.

The researchers observe that the combination treatment results in higher serum levels compared with ezetimibe alone.

Patients aged 7 years and older can now be treated for their cataplexy or excessive daytime sleepiness with the extended-release oral formulation.
This retrospective cohort study provides preliminary evidence for safe removal of mesna from VAdriaC cycles, as the incidence of hemorrhagic cystitis did not increase in patients with Ewing sarcoma who received cyclophosphamide without prophylactic mesna.

RICO is being leveraged in the pharmaceutical industry with significant implications for insurers and public trust.
HER2-mutant NSCLC is associated with poor prognosis. Zongertinib and BAY 2927088 are HER2 tyrosine kinase inhibitors that have activity in NSCLC with manageable toxicity profiles.

The gold standard of testing for food allergies is an oral food challenge test where patients consume the allergen, such as peanut extract, under supervision.

This approval makes foscarbidopa/foslevodopa the first and only subcutaneous 24-hour infusion of levodopa-based therapy for adults with Parkinson disease.

Retail pharmacies benefit from the shift from a volume-centric model to a value-centric approach that focuses on enhancing patient care, reducing pharmacist burnout, and leveraging technology to improve efficiency and expand the scope of pharmacy services.