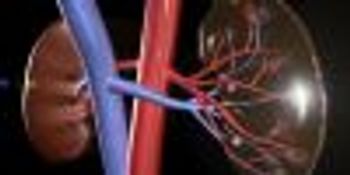
Community pharmacists can identify and educate patients at high risk for acute kidney injury related to use of nonsteroidal anti-inflammatory drugs.

Community pharmacists can identify and educate patients at high risk for acute kidney injury related to use of nonsteroidal anti-inflammatory drugs.
With Americans' use of opioids increasing, a panel from the National Institutes of Health set out to measure the drugs' effectiveness in treating long-term chronic pain, and its report suggested that the risks outweigh the benefits.

It is not inconceivable that a physician who denies requests for opiates may not receive favorable ratings, which may impact their hospital privileges.

Two elderly health care professionals in Houston were recently charged with peddling 1.6 million pain medications over a 3-year period.

Can women who are pregnant be treated safely for acute or chronic severe pain without affecting the unborn child?

The FDA today approved Hospira Inc's diclofenac sodium injection, a proprietary nonsteroidal anti-inflammatory drug analgesic, for the treatment of mild to moderate pain or for the management of moderate to severe pain alone or in combination with opioid analgesics among adults.

John W. Devlin, PharmD, FCCM, FCCP, a clinical pharmacist in the medical ICU at Tufts Medical Center, discusses key considerations for treating delirium in the ICU.

John W. Devlin, PharmD, FCCM, FCCP, a clinical pharmacist in the medical ICU at Tufts Medical Center, discusses what he's learned from using sedation for pain and delirium in critically ill patients.

John W. Devlin, PharmD, FCCM, FCCP, a clinical pharmacist in the medical ICU at Tufts Medical Center, discusses current practice guidelines for pain and delirium that would be important for pharmacists.

Mylan Inc. announced the U.S. launch of its Celecoxib Capsules, 50 mg, 100 mg, 200 mg, and 400 mg, one of the first available generic versions of Pfizer's Celebrex® Capsules, which is indicated for the relief of the signs and symptoms of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, and for the management of acute pain in adults.
A late-stage trial of extended-release hydrocodone bitartrate (Zohydro ER) pointed to a generally safe and well-tolerated response among patients with chronic pain for up to 1 year, according to Zogenix, Inc.
A drug therapy for epilepsy and shingles pain is being voluntarily recalled by its manufacturer due to complaints of empty capsules.

A diverse collective of health care advocacy groups, patient organizations, industry representatives and other stakeholders today announced they have established the Alliance for Balanced Pain Management to support appropriate access to integrated pain management and responsible use of prescription pain medicines with an aim to reduce abuse. AfBPM will work collaboratively to educate, support and advocate on behalf of people affected by pain, both acute and chronic.
Blood testing will soon be painless, quick, accurate, and inexpensive, and patients won't need to go to a lab or doctor's office.

Because orally administered morphine is associated with a significantly greater number of adverse events, ibuprofen is the better choice for relieving pain in children with broken bones.

Roughly two-thirds of emergency department admissions for overdoses involve prescription opioid medications.

It is compulsory for pharmacists to counsel all patients receiving opioids, evaluate the potential benefit of a naloxone reversal device, and contact the opioid prescriber if such a device is deemed appropriate.

Used alongside antidepressants, OTC analgesics and anti-inflammatories purchased from pharmacies may effectively treat patients with depression without increasing their risk of adverse events.

The transdermal buprenorphine manufacturer has launched an awareness campaign to address specific knowledge gaps among pharmacists that pertain to opioids.
Although pharmacies can legally dispense remaining refills of prescriptions for rescheduled hydrocodone combination products submitted prior to October 6, 2014, over the next 6 months, many drug chains have chosen not to honor those refills.

Patients with irritable bowel syndrome cannot block pain signals from the gut as easily as those without the gastrointestinal condition.

In response to the recent article by Gary M. Franklin, MD, MPH, titled "Opioids for chronic non-cancer pain: A position paper of the American Academy of Neurology," we wish to address a number of issues.

All drugs containing the opioid hydrocodone have been reclassified as Schedule II controlled substances, prohibiting pharmacies from recognizing refills and phoned-in prescriptions for those medications.

When quality of wallet takes precedence over quality of life, the healing arts suffer for it.

California joins 5 other states in allowing pharmacists to dispense naloxone without a prescription.