
Compared to other cases, the most recent patient reported more flu-like symptoms, including cough, congestion, watery eyes, and sore throat.

Compared to other cases, the most recent patient reported more flu-like symptoms, including cough, congestion, watery eyes, and sore throat.
Outpatient ASPs remain crucial amid rising antimicrobial resistance.
The prevalence of transmission was higher for those who had contact with children aged less than 5 years and aged 5 to 9 years compared with children aged older than 10.
The data were previously presented at the Multilateral Initiative on Malaria 8th Pan-African Malaria Conference and have been submitted for regulatory review.

Laura Gillespie, PharmD, discusses some of the financial benefits of having a pharmacist focused on antimicrobial stewardship efforts in a health system.

Laura Gillespie, PharmD, discusses the impact of pharmacist-led initiatives on health care–associated C diff rates for health care systems.

Speaker Monica Diaz describes the detrimental impact climate change has on the transmission and increases in infectious disease cases.

The 5-in-1 meningococcal ABCWY vaccine candidate has an assigned Prescription Drug User Fee Act action date of February 14, 2025.

New indications include Staphylococcus aureus bloodstream (SAB) infections, acute bacterial skin and skin structure infections, and community-acquired bacterial pneumonia.
Scientists hypothesize that fecal microbiota transplant restores the recipient’s gut microbiota to an environment that mimics the healthy donor’s microbiota

The test scans whole blood samples from individual human donors for the 5 parasite species that can cause malaria in humans and detects Plasmodium RNA and DNA.

As data continue to develop in this arena, β-lactam therapeutic drug monitoring is likely to become more routine, as it has with drugs like vancomycin and the aminoglycosides.
Merck will also conduct clinical trials in both males and females to evaluate the efficacy of a single dose of their current human papillomavirus vaccine compared with the approved 3-dose regimen.
Study results indicate early swap to oral antibiotics in Staphylococcus aureus bacteremia is just as effective.

GBS can cause transcriptional adaptations, which may increase risk of poor neonate outcomes.
The regimen, which was used as second-line treatment in patients with drug-resistant TB, had a similar microbiological response to those with drug-sensitive TB taking first-line regimens.
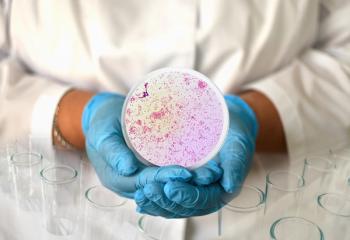
Gepotidacin was compared with the combination of ceftriaxone and azithromycin in adolescent and adult patients with uncomplicated urogenital gonorrhea.

Malaria has a large global burden, affecting hundreds of millions of people each year.
Targeted use of ultraviolet light and sporicidal treatment can reduce disease transmission alongside existing interventions.

The researchers made a connection between positive outcomes for infectious disease patients, higher reimbursement class, and the presence of pharmacists in care teams.
Investigators found high incidences of non-susceptibility with penicillin and increasing resistance to macrolides for pneumococcal diseases.
Investigators from the University of Liverpool tested the effect of meropenem, an antibiotic used for hospital-acquired pneumonia, to determine how resistance emerges.

Creating a free app to assist in vancomycin dosing proved to be an entertaining and enlightening journey.
Older adults without a Part D plan face costs ranging from $180 to $295 per shot.