
A New York City physician who became infected with Ebola after treating patients in West Africa was released from the hospital after being declared virus free.

A New York City physician who became infected with Ebola after treating patients in West Africa was released from the hospital after being declared virus free.

A study exploring Merck's experimental hepatitis C virus treatment has provided proof-of-concept for a 6- to 8-week course of triple therapy.

A potential Ebola outbreak in Senegal was snuffed out thanks in part to an innovative text-messaging program originally intended to help patients with diabetes manage their disease.

The World Health Organization is criticizing the drug industry for delaying the development of an Ebola vaccine.
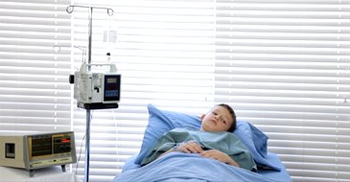
The severe respiratory illness that infected more than 1000 children across the United States is starting to wane.

Astellas today announced that the FDA has granted orphan drug designation to isavuconazole for the treatment of invasive candidiasis, one of the most frequent invasive fungal infections detected in critically ill patients and a common cause of bloodstream infections.
Could the key to fighting modern illnesses lie in a disease that was an epidemic centuries ago?

New recommendations for the removal of protective gear highlight the need for enhanced technologies.

The FDA today approved the first vaccine licensed in the United States to prevent invasive serogroup B meningococcal disease in patients aged 10 to 25 years.

Federal officials are questioning state policies that mandate a 21-day quarantine of health care workers who treat Ebola patients.
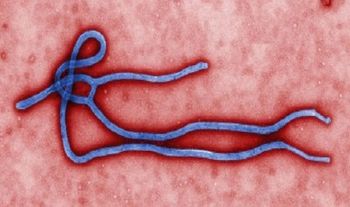
Three individuals who were in close contact with an Ebola patient have been placed under quarantine.

Learn about the strains involved in current and past Ebola epidemics, features of the virus, information about aerosolized spread, and treatments in development.

Progress in the fight against an Ebola outbreak has been made on 2 fronts.

A hospital employee may have been in contact with clinical specimens from a deceased Ebola patient.

The second nurse to become infected with the Ebola virus after treating the index patient in Texas should not have been allowed to board a flight from Ohio earlier this week.

A second health care worker who tested positive for the Ebola virus traveled by air the day before her symptoms appeared.
The vaccine that prevents illness and death from pneumococcal infection also combats severe antibiotic-resistant disease in young children.

Investigators are working to resolve how a health care worker in Texas contracted the Ebola virus while caring for an infected patient.

Roughly half of all patients hospitalized between May 2011 and September 2011 received at least 1 antimicrobial drug.

Five US airports that receive the majority of travelers from West African nations will add new layers of entry screening.
Although many policymakers recommend universal screening for methicillin-resistant Staphylococcus aureus infection, individual hospitals may lack the funding to execute it.

World Health Organization officials are stressing that clinical trials on Ebola vaccine candidates need to be expedited.

Parents who know about the human papillomavirus are not more likely to get their adolescent daughters vaccinated.

We will soon have a wartime economy against an enemy that we can't even see.