HER2 Breast Cancer
Latest News
Latest Videos

CME Content
More News
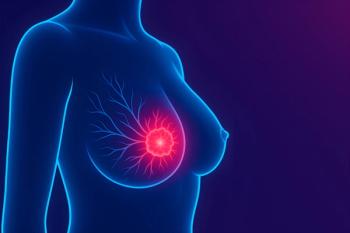
Study Finds HER2-Negative Outperforms HER2-Low in Neoadjuvant Chemotherapy Response in Breast Cancer
Research reveals HER2-negative breast cancer shows higher pCR rates than HER2-low, influencing neoadjuvant chemotherapy strategies for early-stage patients.
Combination therapy with trastuzumab emtansine and trastuzumab significantly enhances survival rates in HER2-positive early breast cancer, outperforming trastuzumab alone.
Dalpiciclib combined with endocrine therapy significantly enhances progression-free survival in HR+/HER2– breast cancer, as shown in the DAWNA-A trial.
Radiation or Immune-Modulating Drugs Could Mitigate Resistance to CDK4/6 Inhibitors in Breast Cancer
Research reveals that combining radiotherapy or immune-modulating drugs with CDK4/6 inhibitors enhances treatment efficacy in HR+/HER2– breast cancer.

The data revealed that only 75% of patients receive HER2-targeting therapies.

Ribociclib in combination with a nonsteroidal aromatase inhibitor (NSAI) improved invasive disease-free survival (iDFS) while allowing for better quality of life in hormone receptor-positive (HR+)/human epidermal growth receptor 2-negative (HER2−) early breast cancer.
About 64.1% of patients receiving taxane, trastuzumab, and pertuzumab (THP) without carboplatin had pathological complete response rates (pCRs).
Vepdegestrant was the first PROTAC to be evaluated in a phase 3 clinical trial.
Compared with standard of care, camizestrant in combination with a CDK4/6 inhibitor shows promise in enhancing progression-free survival (PFS) for patients with advanced breast cancer.

Experts share insights on patient discussions and adverse effect management strategies in HER2 breast cancer.
Compared with fulvestrant, vepdegestrant significantly extended progression-free survival and improved response rates.
Zongertinib shows significant benefits in managing symptoms and improving quality of life for patients with HER2-mutated non-small cell lung cancer.
Hope Rugo, MD, discusses a multi-institutional real-world analysis showing that rechallenging trastuzumab deruxtecan after grade 1 interstitial lung disease (ILD) is generally safe and effective.

This segment takes a deeper dive into toxicity management—focusing not just on what to monitor, but how institutions are putting these protocols into action in real-world clinical settings.
Trastuzumab deruxtecan (T-DXd) yielded favorable outcomes and improved survival rates.
The data were presented at the ESMO Breast Cancer 2025 Annual Meeting.
Studies suggest that prolonged activation of MUC1-C encourages disease progression.

Pharmacists compare real-world practice data with clinical trial results and discuss supportive care options for patients.
New data reveals that combining pertuzumab with trastuzumab significantly improves survival rates in HER2+ breast cancer patients, reducing death risk by 17%.

Explore the latest insights on emerging antibody drug conjugates for HER2 breast cancer and real-world management strategies.
Trastuzumab deruxtecan was safe and efficacious prior to treatment with paclitaxel, trastuzumab, and pertuzumab.

This installment of our series on ADCs and HER2 breast cancer offers key insights about novel agents in clinical trials and management of ADC therapies.

Pharmacists discuss how drug design impacts drug efficacy and indications for HER2-low and -ultra-low breast cancer.
Pyrotinib is a tyrosine kinase inhibitor that is independently developed in China.
Patients in both arms achieved statistically significant rates of compliance up to 97%.