Pacritinib is a JAK2/FLT3 inhibitor for the treatment of patients with myelofibrosis and severe thrombocytopenia defined as platelet counts of less than 50,000 μL.

Pacritinib is a JAK2/FLT3 inhibitor for the treatment of patients with myelofibrosis and severe thrombocytopenia defined as platelet counts of less than 50,000 μL.
In the absence of a direct head-to-head clinical trial, study may help inform treatment selections for stem cell transplant-ineligible patients.

KG is a female patient aged 44 years who came to the pharmacy with a prescription that the pharmacist recognized is the treatment regimen for a new blood clot.

Following the release of a new report by the HHS acknowledging pharmacists as integral members of the hypertension care team, the NACDS released a statement on the importance of this report.
Institutions should consider optimal targets, testing volumes, and notification and reporting practices when making their choice.

The FDA has approved mepolizumab for the treatment of hypereosinophilic syndrome.
September has been designated as National Sickle Cell Awareness Month for the continued fostering of education surrounding sickle cell disease management.
Researchers at the Rensselaer Polytechnic Institute recently published a study on the use of the FDA-approved anticoagulant heparin in patients with COVID-19.

Azacitidine showed an overall survival benefit among patients with acute myeloid leukemia compared with a placebo.
Current advances in the analysis of the mutational status of different genes are providing investigators insights into new treatment pathways that can improve outcomes for patients.
A new medication for certain rare blood cancers has now become available through a limited distribution with AllianceRx Walgreens Prime.
Targeted combination therapy found to improve overall survival with rapid and durable treatment responses in patients with acute myeloid leukemia.
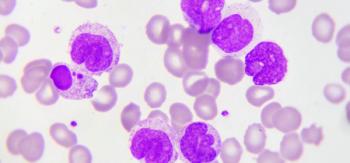
CAR T-cell therapy involves collecting a patient’s blood, extracting the T cells within the blood, and returning the remaining blood back into the body, a process called leukapheresis.

The FDA has issued an emergency use authorization for the use of convalescent plasma in the treatment of COVID-19.

The FDA has approved an expansion of the prescribing information for carfilzomib to include its use with daratumumab and dexamethasone for the treatment of relapsed or refractory multiple myeloma.

Belantamab mafodotin-blmf is the first anti-BCMA therapy approved by the FDA for the treatment of patients with relapsed or refractory multiple myeloma who received at least 4 prior therapies.

As the most accessible member of the health care team, the pharmacist can provide patients with cancer with the information and support necessary to get through what might be the most challenging time of their lives.
“These new therapies allow for treatment without some of the traditional chemotherapy agents.”

The investigators found that patients with preexisting neutropenia could be managed on venetoclax, although use of G-CSF would likely also be required in conjunction with the treatment.

Brexucabtagene autoleucel (Tecartus, Kite Pharma) is indicated for adult patients diagnosed with mantle cell lymphoma (MCL) who have not responded to or who have relapsed following other kinds of treatment.
The review also summarized proposed mechanisms behind these wide-ranging systemic effects and provided clinical guidance for physicians, according to a press release.
The test is intended to determine whether someone is currently infected with COVID-19 and if they have been infected in the past.

Caroline Bernal-Silva, a patient spokesperson for the National Blood Clot Alliance, discusses a recent survey regarding patients’ fears of a life-threatening bleeding event while taking anticoagulant medications.

According to new research published in Clinical and Translational Immunology, a new cancer vaccine is ready for human trials following its success in preclinical studies.
The von Willebrand factor may be the reason why coronavirus disease 2019 presents itself so differently in each person.