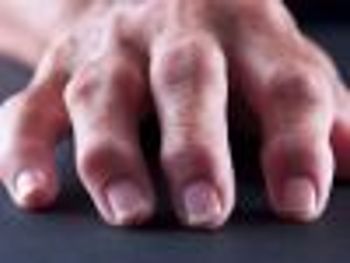
In a retrospective analysis, researchers concluded that rheumatoid arthritis patients had better outcomes when they filled their prescriptions through a mail-order specialty pharmacy rather than a retail pharmacy.

In a retrospective analysis, researchers concluded that rheumatoid arthritis patients had better outcomes when they filled their prescriptions through a mail-order specialty pharmacy rather than a retail pharmacy.

New recommendations from the task force say that all Americans aged 15 to 64 years should get an HIV test regardless of their risk for contracting the virus.

In an exclusive interview with Specialty Pharmacy Times, Albert Thigpen, Senior Vice President of Pharmacy Operations & Industry Relations at Catamaran Corporation, spoke about his distinct responsibilities surrounding the management of pharmaceutical manufacturers, Catamaran's 3 mail order pharmacies, and BriovaRx's 11 specialty pharmacies.

A recent study featured in the American Journal of Pharmacy Benefits examines the role of patient out-of-pocket cost on adherence to disease-modifying therapies for multiple sclerosis.

At a meeting hosted by the American Journal of Managed Care, key figures in the managed care industry discussed innovative payment models being utilized by payers and providers.

Pfizer's Xeljanz (tofacitinib) is the first Janus kinase inhibitor approved for rheumatoid arthritis and the first oral biologic within the RA class. Dina Rufo of GlobalData discusses the factors that will affect uptake of the drug.

Community Specialty Pharmacy Network, Inc (CSPN), recently announced a distribution agreement with Dara BioSciences for Soltamox (tamoxifen citrate), a nonsteroidal antiestrogen for the treatment of patients with breast cancer.

Diplomat Specialty Pharmacy announced that Jennifer Cretu has been promoted to vice president of information technology and pharmacy executive Thomas Michaels has joined the company as vice president of sales and marketing.

The Kroger Co today announced it will acquire the outstanding shares of Axium Pharmacy Holdings, Inc, a leading specialty pharmacy, to create a merger of the 2 companies.

According to a report by Elsevier's Business Intelligence, UnitedHealthcare has announced that pharmacies that are participating in its Designated Specialty Pharmacy network will no longer redeem manufacturer-sponsored coupons.
Sanofi slashed the price of colorectal cancer drug Zaltrap (ziv-aflibercept) in half after 2 oncologists from Memorial Sloan-Kettering Cancer Center publicly balked at the drug's $11,000 price tag in an op-ed in The New York Times.

Value-added services for specialty drugs can help distributors support otherwise slim profit margins.

New legislation would allow the FDA to track the volume and distribution of drugs produced at compounding pharmacies, increase the transparency of compounded drugs, and institute a waiver system in times of public need.

The FDA approved Xeljanz (tofacitinib) to treat adults with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response to, or who are intolerant of, methotrexate.

Diplomat Specialty Pharmacy, the nation's largest privately held specialty pharmacy company servicing the needs of patients and physicians nationwide, is on target to meet its long-term growth plan with the announcement that it has an immediate need to fill 100 full-time jobs.

Considering the delicate nature of specialty pharmaceuticals, specialty pharmacies must take measures to ensure they have a crisis communication plan in place in the event of a natural disaster or an emergency.

Cardinal Health Specialty Solutions announced today that its specialty pharmacy, OncoSourceRx, has been awarded Specialty Pharmacy Accreditation from URAC, a Washington, DC-based health care accrediting organization that establishes quality standards for the health care industry.

The American Society of Clinical Oncology's list of the top 5 common oncology treatments or procedures that "lack sufficient evidence for widespread use" should be considered when discussing the rising cost of cancer care.

Avella Specialty Pharmacy, under an agreement with DARA BioSciences, Inc, will be a distribution source for Soltamox, the first and only FDA-approved oral liquid formulation of tamoxifen citrate for the prevention and treatment of breast cancer.

CVS Caremark announced the appointment of Alan Lotvin, MD, to the position of executive vice president, specialty pharmacy.

In this Medscape One-on-One video, former FDA Commissioner and former director of the National Cancer Institute Andrew von Eschenbach, MD, suggests using adaptive trial design to test drugs rather than a stepwise approach.
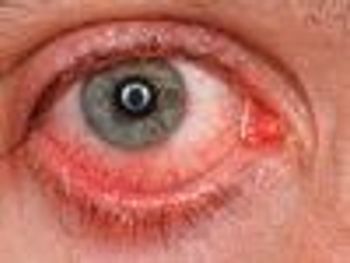
On October 17, the FDA approved Jetrea (ocriplasmin), the first drug approved to treat an eye condition called symptomatic vitreomacular adhesion (VMA).
In a session at the 2012 Academy of Managed Care Pharmacy Educational Conference, Aimee Tharaldson, PharmD, identified the top specialty drugs in the pipeline and highlighted the most recent therapeutic trends within each medication class.
A new study carried out by Blue Cross and Blue Shield of Minnesota and PBM Prime Therapeutics forecasts that multiple sclerosis specialty drug treatment cost will exceed $50,000 per person per year in 2016.
A recent outbreak of meningitis tied to a compounded drug raises concerns about the scope of the practice of pharmacy compounding and prompts legislators to push for increased FDA authority over these products.
Therapies for chronic conditions are increasingly being subjected to utilization management tools, according to ICORE Healthcare's 2011 Medical Pharmacy & Oncology Trend Report.

In this video from The American Journal of Pharmacy Benefits, Aimee Tharaldson, PharmD, discusses some of the current trends in the specialty pharmacy market and notes that the pipeline for cancer drugs is likely to be the most robust.
A free source of evidence-based information for health care professionals and for researchers studying liver injury associated with prescription and over-the-counter drugs, herbals, and dietary supplements is now available from the National Institutes of Health.
Celgene Corporation today announced the FDA has approved Abraxane (paclitaxel protein-bound particles for injectable suspension) (albumin-bound) as a first-line treatment for locally advanced or metastatic non-small cell lung cancer, in combination with carboplatin, in patients who are not candidates for curative surgery or radiation therapy.
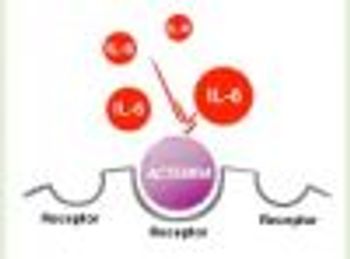
Genentech, Inc, a member of the Roche Group, today announced that the FDA has expanded the approved indication for Actemra (tocilizumab) for the treatment of adults with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response to one or more disease-modifying antirheumatic drugs (DMARDs).