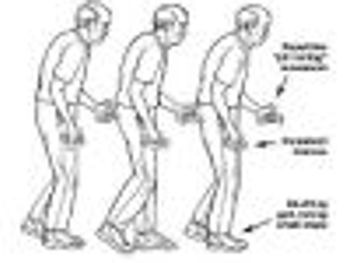
Modifying the activity of the gene that becomes mutated in Parkinson's disease may alter disease progression.

Modifying the activity of the gene that becomes mutated in Parkinson's disease may alter disease progression.

H.D. Smith, among the nation's largest pharmaceutical wholesalers, recently announced a 3-year prime vendor agreement with PharmacyGPO, a buying group that services independent community pharmacies across the United States.
More than 75 million dollars worth of stolen medications was recently recovered from a Florida warehouse.

FDA releases a safety announcement saying that patients treated with Revlimid (lenalidomide) may be at an increased risk of developing new types of cancer.

Walgreen Co. announced that it has completed its acquisition of certain assets of BioScrip, Inc's community specialty pharmacies and centralized specialty and mail service pharmacy businesses.

Access to food and shelter, rather than viral load count, is a better predictor of overall health among homeless HIV-infected men, according to a recent study.

Facing new agents and rising costs, payers continue to tighten their management of oncology.

Walgreens Specialty Pharmacy is adding 6 medications to its oral oncology cycle management program, in a significant expansion of the comprehensive treatment and support program it provides to benefit patients, physicians and payers.

The FDA approved Elelyso (taliglucerase alfa), the first drug ever produced in a genetically engineered plant cell.
Women with autoimmune disorders such as RA generally have fewer children than they would like to have, according to a survey that appeared recently in Arthritis Care and Research.

Novartis Pharmaceuticals Corporation announced that the US Food and Drug Administration (FDA) approved Afinitor (everolimus) tablets for the treatment of adult patients with kidney tumors known as renal angiomyolipomas and tuberous sclerosis complex (TSC), who do not require immediate surgery.
The United States Food and Drug Administration approved Votrient (pazopanib) to treat patients with advanced soft tissue sarcoma who have previously received chemotherapy. Soft tissue sarcoma is a cancer that begins in the muscle, fat, fibrous tissue, and other tissues.
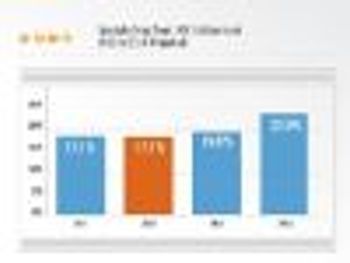
The specialty drug trend is projected to rise 17.1% in 2012, 19% in 2013, and 22% in 2014.

URAC developed a specialty pharmacy accreditation program because it is one of the key distribution channels for pharmacy benefit management, and it extends URAC's consumer protection and empowerment efforts in managed care pharmacy.

Managed Health Care Associates, Inc (MHA), announced the launch of MHA Clinical Therapy Management (CTM), a proprietary application used to assist pharmacies in the clinical management of patients utilizing specialty therapies.

Presenters from the Academy of Managed Care Pharmacy (AMCP) 24th Annual Meeting recently discussed the newest updates in specialty pharmacy.
Express Scripts recently announced that their new Drug Trend Report is available for download.

BioPlus Specialty Pharmacy has launched TAP App, a new proprietary, secure web-based portal that connects physicians with extensive information about their patients who are being treated through BioPlus's specialty pharmacy.

Diplomat Specialty Pharmacy, the nation's largest, privately owned specialty pharmacy, announced today that Gary Rice, RPh, a veteran specialty pharmacy executive, has joined Diplomat's executive team as vice president of clinical services.
AnaptysBio, Inc, a privately held therapeutic antibody company, recently announced a strategic partnership with Celgene Corporation to develop novel antibodies against multiple therapeutic targets.
New combination pills set to hit the market are expected to be more expensive than prior treatments.
FDA lab tests have confirmed that a counterfeit version of Roche's Altuzan 400mg/16ml (bevacizumab), an injectable cancer medication, found in the United States contains no active ingredient.

Novartis and the Broad Institute have developed a cancer cell line encyclopedia that catalogues the genetic and molecular profiles of almost 1000 human cancer cell lines used in drug research and development.

The FTC's approval of the highly publicized merger may depend on the divestiture of some of Medco's assets.
The FDA today approved Omontys (peginesatide) to treat anemia, a condition in which the body does not have enough healthy red blood cells, in adult dialysis patients who have chronic kidney disease (CKD).
ARIAD Pharmaceuticals, Inc today announced its schedule of preclinical data presentations to be made at the American Association for Cancer Research (AACR) Annual Meeting 2012, taking place Saturday, March 31 through Wednesday, April 4, 2012, in Chicago.
Cost-sharing practices that limit access to vital medications for West Virginians with serious health conditions may soon be alleviated thanks to a bill just introduced in Congress. The bi-partisan legislation, supported by the American College of Rheumatology, the Arthritis Foundation, and other medical and patient groups, has been introduced by Rep David B. McKinley (R,WV) and Rep Lois Capps (D,CA).

GlaxoSmithKline announced today that the Oncologic Drugs Advisory Committee (ODAC) to the FDA voted 11 to 2 that evidence from clinical studies support a favorable benefit–risk assessment for use of Votrient in treating patients with advanced soft tissue sarcoma who have received prior chemotherapy.

A new letter goes out to the FTC requesting that their Express Scripts-Medco review include a careful assessment of specialty pharmacy and exclusive distribution contracts.
Randell "RJ" Correia, PharmD, Senior Vice President, Pharmacy Services, Optum Rx, addresses the rapid rise of specialty pharmacy spending. Dr. Correia discusses the possible complications health reform exchanges may cause and the impact they will have on the future of pharmacy benefit drug management.