Updates to this invaluable industry guide include new portions pertaining to specialty pharmaceuticals, comparative effectiveness research, and companion diagnostics tests, as well as a new definition for the term "specialty pharmacy."

Updates to this invaluable industry guide include new portions pertaining to specialty pharmaceuticals, comparative effectiveness research, and companion diagnostics tests, as well as a new definition for the term "specialty pharmacy."

Research proceedings from the 2nd Annual Foundation for Managed Care Pharmacy (FMCP) Research Symposium: Contemporary Applications for Specialty Pharmacy are now available online.

McKesson Specialty Health and The US Oncology Network today announced that Marcus Neubauer, MD, has accepted the position of medical director, Oncology Services for McKesson Specialty Health, to continue his work on pathways development.
The FDA today approved a new use of Gleevec (imatinib) to treat children newly diagnosed with Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL).
The Arizona Chapter of The Leukemia & Lymphoma Society and Avella Specialty Pharmacy announce they will present a day of discussion about the latest advancements, trends, and management of oral chemotherapy medications on February 9, 2013.

The FDA today expanded the approved use of Exjade (deferasirox) to treat patients 10 years and older who have chronic iron overload resulting from a genetic blood disorder called nontransfusion-dependent thalassemia (NTDT).

A new report from The Analysis Group created with PhRMA's support highlights how biopharmaceutical industry strategy is shifting from a "me-too" to a "me-first" paradigm.
H. D. Smith, among the nation's largest pharmaceutical wholesalers, today announced the establishment of H. D. Smith Specialty Solutions, an organization structured to provide focus and alignment around specialty and traditional brand products, solutions and services.

H. Lundbeck A/S (Lundbeck) has appointed Barbara Jaszewski as vice president of global pricing and market access. The move strengthens the company's capacity to bring drugs to market effectively prior to the launch of 3 new drugs in the coming 12 months.

Pharmacy Times and Parata Systems, founding sponsors of the Next-Generation Pharmacistâ„¢ awards-the industry's leading national awards program-are pleased to announce the official opening of nominations for the 2013 program.

The class action lawsuit against Anthem Blue Cross alleges their policy mandating that patients use a mail-order specialty pharmacy discriminates against HIV patients.

BioPlus Specialty Pharmacy selected as one of the limited number of specialty pharmacies with access to the injectable medication Vivitrol (naltrexone).

AIDS Healthcare Foundation (AHF), announced that following its August 2012 acquisition of MOMS Pharmacy, a specialty pharmacy focused exclusively on providing medications and support services to people living with HIV/AIDS, it is fully incorporating and rebranding the chain into its own AHF Pharmacy brand.
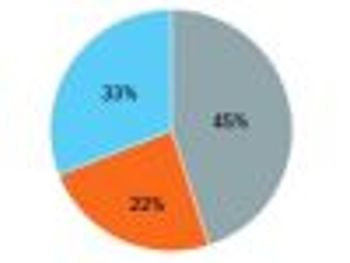
Forecasted spend amounts in the specialty category often do not account for the spend that occurs under the medical benefit in the outpatient hospital setting.

H. D. Smith, among the nation's largest pharmaceutical wholesalers, today announced plans to secure majority ownership in Triplefin, a privately held reimbursement, patient assistance, and pharmaceutical brand support services company, headquartered in Cincinnati, Ohio.

Xcenda and US Bioservices are scheduled to host a summit focusing on the increasingly complex relationships between payers, manufacturers, and specialty pharmacies.

Uptake of oral nucleoside/nucleotide analogues will increase from 2011 to 2021, according to new research from Decision Resources.
The Breakthrough Therapy initiative, which is part of the 2012 Food and Drug Administration Safety and Innovation Act, will bring promising treatments more quickly through an expedited review process.
Telik, Inc was recently notified that its product candidate, Telintra (ezatiostat HCL) has been granted orphan drug designation by theFDA for the treatment of myelodysplastic syndrome (MDS).

Professor Dusheiko talks about managing HCV patients with cirrhosis.

Here, UCSF's Annie Luetkemeyer talks about new treatments for hepatitis C and what they will do for people with infections.

FHI 360 President Emeritus Ward Cates discusses the HIV treatment cascade. This model aims to bring us closer to the end of AIDS by addressing the gaps in the HIV diagnosis and treatment system currently in place.

Arun K. Ghosh. professor of organic and medicinal chemistry at Purdue University, talks with Hiroaki Mitsuya, Head of Experimental Retrovirology Section at the National Cancer Institute, about HIV drug development.

Dr. Paul Richardson discusses promising drugs in the pipeline for the treatment of myeloma and new combination treatments using older drugs.
This video describes the use of targeted therapies for the treatment of multiple myeloma.

Landmark legislation requiring the National Cancer Institute to ramp up their research efforts on cancers with very low survival rates was signed into law early this month.

Stephen Vogt, PharmD, endeavors to create personalized treatment strategies for each patient.

National Association of Chain Drug Stores announced it has just added a "Developing Specialty Pharmacy" session to its program lineup for the 2013 Regional Chain Conference, which will take place February 3-5, 2013, in Fort Lauderdale, Florida.
Payers look to hepatitis C and do not see the value of credentialing bodies in specialty pharmacy, according to The Zitter Group's Fall 2012 Managed Care Biologics and Injectables Index.

Hospice Pharmacia (HP), a service of excelleRx, Inc, an Omnicare Specialty Care Group company and provider of medication management and pharmacy services, recently announced a new partnership with AccentCare, Inc, a leading multistate provider of hospice services.