
New research from Medco Health Solutions Inc shows that many patients using oral cancer medications are also on other drugs that could reduce the effectiveness of the cancer treatment or increase its toxicity.

New research from Medco Health Solutions Inc shows that many patients using oral cancer medications are also on other drugs that could reduce the effectiveness of the cancer treatment or increase its toxicity.

AmerisourceBergen acquires World Courier Group, Inc, and expands its international market reach for specialty medications.

69% of the employer groups studied in this report want more education on specialty pharmacy services.

Managed Health Care Associates, Inc. (MHA), a leading health care service company focused on alternate site health care providers, announced the completion of its fifth annual Independent Long Term Care Member Study, the results of which were released at the 2012 MHA Business Summit, March 2, 2012 in Las Vegas, Nevada.
The Infectious Diseases Society of America (IDSA) proposed a new pathway, the "Special Population Limited Medical Use (SPLMU)" mechanism, to provide an important new approval option for companies interested in developing drugs to treat patients with serious infections where few or no treatment options exist.

Prime Therapeutics and EMD Serono entered into an agreement where patient adherence and health outcomes data determine whether or not rebates are given for a drug.

Two companies focused on delivering preventative care to individuals looking to fight chronic disease with nutrition, fitness, and bioidentical hormone therapy-Diplomat Specialty Pharmacy and Ageology-announced that they have formed a strategic alliance.

The Independent Specialty Pharmacy Coalition (ISPC), a national group of community based specialty pharmacies, recently sent a letter to the Federal Trade Commission (FTC), urging the Commission to protect consumers and restrict anticompetitive conduct if it chooses to approve the proposed merger between Express Scripts, Inc (ESI) and Medco Health Solutions.
A strategy in the development of an HIV vaccine relies on a mathematical technique historically used to predict changes in the stock market.

New research suggests that using a nanorobot may be a promising method to deliver drugs directly to their targets.
Yesterday Biogen Idec announced the company has submitted a New Drug Application (NDA) to the FDA for marketing approval of BG-12 (dimethyl fumarate), the company's oral therapeutic candidate for the treatment of multiple sclerosis (MS).

AIDS Action Committee adds onsite specialty HIV MOMS Pharmacy to their new drop-in center.
New data unveiled at the 17th Annual Pharmacy Benefit Management Institute Conference shows patients, health plans and employer groups can save as much as 60 percent through site-of-care optimization.

The switch from ICD-9 diagnosis and procedure codes to ICD-10 codes is postponed for certain health care entities to an undetermined date.

A new plan to eliminate waste, promote adherence, and coordinate coverage of expensive specialty medications is announced by Medco.

A severe shortage of the key medication for childhood leukemia appears to have been averted, but the problem is likely to reemerge in the future.

AstraZeneca recently announced its first direct-to-patient program offering patients with prescriptions for Arimidex (anastrozole) the opportunity to receive their medication for $40 a month through Express Scripts, a specialty pharmacy provider.

As health care costs continue to increase, and with high-cost specialty products making up a significant portion of the pipeline, the role of the Specialty Pharmacy (SP) continues to grow and influence health care decisions across multiple stakeholders.
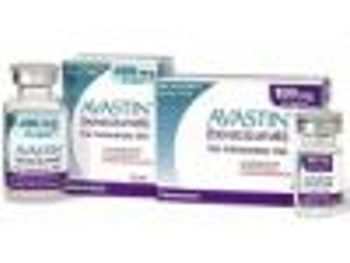
The FDA released a statement on February 14, 2012, warning pharmacies, medical practices, and patients that a counterfeit version of the drug Avastin-an injectable drug used to treat cancer of the colon, lung, kidney, and brain-has been purchased and used by some medical practices in the United States.
The Centers for Disease Control and Prevention (CDC) recently added to their list of HIV resources in the Electronic HIV/AIDS Prevention (e-Hap) portion of their website, a section which is produced with content from CDC's Division of HIV/AIDS prevention (DHAP).
The U.S. Food and Drug Administration today issued three draft guidance documents on biosimilar product development to assist industry in developing such products in the United States.

The FDA sent out a "Drug Safety Communication" from their Division of Drug Information on February 9, 2012, listing the important new drug interactions between Victrelis (boceprevir) and ritonavir-boosted HIV protease inhibitor drugs.

Show your support for World Cancer Day on February 4, 2012, by pledging to make healthier choices through the use of social media applications dedicated to the cause.

Acquisition strengthens Walgreens retail specialty pharmacy offering with access to additional drug therapies and services.

Today, Erivedge (vismodegib) was approved by the U.S. Food and Drug Administration to treat adult patients with basal cell carcinoma, the most common type of skin cancer.
The FDA points out the risk factors associated with using unapproved cancer injectables and issues new warnings to health care providers.

Serving a growing market, the new Specialty Pharmacy Times Website provides industry news, current clinical insights, and specialty trends and practices.

New rules will require physicians to report their financial transactions with drug companies through a public database.
Florida's new pain management law sparks a discussion about restricting access to controlled substances.

Manufactures are faced with multiple challenges as the FDA imposes far-reaching requirements for their production.