
NVG-111 is a humanized, tandem single-chain variable fragment ROR1 x CD3 BiTE being evaluated in relapsed/refractory chronic lymphocytic leukemia and mantle cell lymphoma.

NVG-111 is a humanized, tandem single-chain variable fragment ROR1 x CD3 BiTE being evaluated in relapsed/refractory chronic lymphocytic leukemia and mantle cell lymphoma.

Eflapegrastism-xnst injection (Rolvedon, Spectrum Pharmaceuticals) is indicated to lower the incidence of infection, as demonstrated by febrile neutropenia, in adult patients with non-myeloid malignancies.

Researchers found differences in the variable importance of cancer mortality risk factors depending on an individual’s geographical region.

Prior authorizations have delayed time to treatment for patients and can directly impact clinical outcomes.
New research presented at ESMO Congress found that certain patients with advanced melanoma administered a combination of cemiplimab with fianlimab experienced a median progression-free survival of 2 years.
Researchers at the European Society for Medical Oncology Congress presented findings that show osimertinib may clinically reduce the risk of lung cancer recurrence.

Research suggests that rucaparib can increase progression-free survival by more than a year in women with advanced ovarian cancer.

Trastuzumab deruxtecan produces a confirmed objective response rate of 53.8% and 42.9% in the 5.4 mg/kg arm and 6.4 mg/kg treatment arms, respectively, among patients with HER2 mutant non-small cell lung cancer.

An estimated 31.4% of individuals with non-squamous non–small cell lung cancer treated with the combination were alive at 3 years compared to 17.3% for those on chemotherapy alone.

Results demonstrated a 64.5% confirmed objective response rate in patients treated with the investigational combination.

Ribociclib with endocrine therapy was found to increase the median overall survival of patients with HR+/HER2- advanced breast cancer with visceral metastases to nearly 5 years.
Data presented at the 2022 ESMO congress show poziotinib has high activity in both treatment-naïve and previously treated patients with non-small cell lung cancer.

Prescribers signed a letter expressing concerns about recommendations for restrictions on compounded hormones, which millions of women and other patient populations rely on.
Fruquintinib reduced the risk of death in patients with advanced metastatic colorectal cancer by 34% and led to longer overall survival than among those given placebo.

Scientists may be able to minimize adverse effects while gaining a better understanding of psychedelic medicines' efficacy and durability.
Study suggests that myeloma patient survival after BCMA-targeted T-cell therapy could be determined by microenvironment factors.

The biosimilar is expected to be launched as a prefilled syringe in early 2023.

One-third of participants who have used prescriptions think that mail-order pharmacies would help reduce drug costs, according to HealthCare.com.

Results from the TOPAZ-1 phase 3 trial showed that the combination reduced risk of death by 20% versus chemotherapy alone.

AI-based technologies are increasingly being used for things such as virtual screening, physics-based biological activity assessment, and drug crystal-structure prediction.
The 2022 Biosimilar Survey signaled that most pharmacists are willing to incorporate biosimilars into their formularies, but certain factors are preventing widespread adoption.

DNA and RNA sequencing could provide insight about resistance to chemotherapy in patients suffering from triple-negative breast cancer.
Test can evaluate whether a patient with lymphoma will respond to CAR T-cell therapy, which could lead to better and longer lasting treatment options.
The phase 2 AXIOMATIC trials assessing milvexian met its primary endpoints, with researchers anticipating a phase 3 trial to come within the year.

An overview of the legal and business tools available to community, specialty, hospital, and health-system pharmacies in seeking to maintain payer network access and combat abusive practices.

The FDA approval makes olipudase alfa the first approved drug to target the physiological symptoms of the disease.
Vertical and horizontal consolidation has had a significant impact on retail, specialty, and health-system pharmacies.
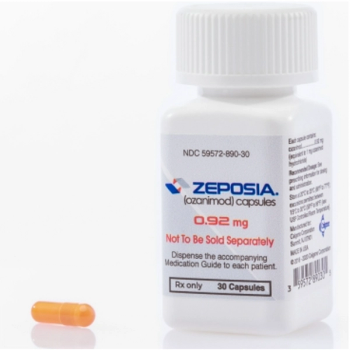
Ozanimod (Zeposia) is indicated for the treatment of multiple sclerosis and moderately to severely active ulcerative colitis in adults.

Atezolizumab meets its primary endpoint of overall survival for patients with resected non-small cell lung cancer across most subgroups.
Study evaluates efficacy, immunogenicity, safety of the product to eculizumab in patients with paroxysmal nocturnal hemoglobinuria.