
Abrocitinib (Cibinqo) is a Janus kinase inhibitor indicated for the treatment of adults with refractory moderate-to-severe atopic dermatitis.

Abrocitinib (Cibinqo) is a Janus kinase inhibitor indicated for the treatment of adults with refractory moderate-to-severe atopic dermatitis.
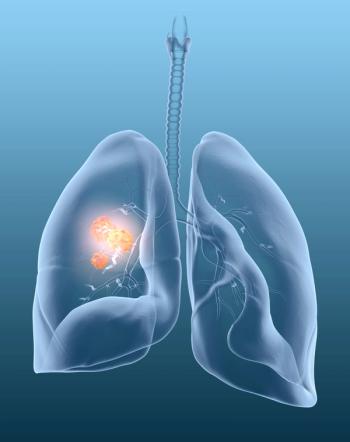
Clinical data from phase 1/2 LIBRETTO trial show evidence of meaningful clinical outcomes for patients, including those with difficult-to-treat brain metastases.
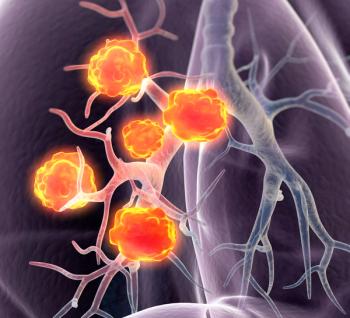
Sotorasib demonstrates clinical benefit and prolonged tumor response seen, with a 40.7% objective response rate.
Ravulizumab-cwvz (Ultomiris) produced statistically significant improvements in measures of functional activity, muscle strength, and quality of life in patients with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis.

Biosimilars could save consumers more than $100 billion in the coming years.

Britny Brown, PharmD, BCOP, vice chair of the Hematology/Oncology Pharmacy Association (HOPA) DEI Task Force and clinical assistant professor at the University of Rhode Island College of Pharmacy, discusses the role of the task force within HOPA and its impact on the field more broadly.

Maralixibat (Livmarli) is an ileal bile acid transporter inhibitor indicated for the treatment of cholestatic pruritus in patients with Alagille syndrome 1 year of age and older.
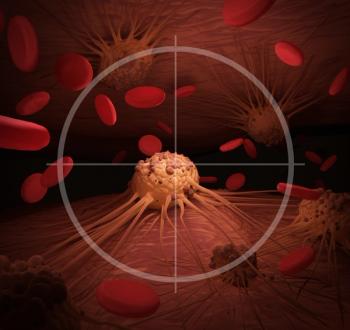
Cutaneous squamous cell carcinoma should be diagnosed early and treated promptly to avoid complications.
In studies of rats, results show that supplementation preserves skeletal muscle after patients take the chemotherapy medication doxorubicin.

Peptide-targeted radionuclide therapy FAP-2286 (Clovis Oncology) showed potent affinity for human fibroblast activation protein by biochemical and cell-based assays.

On average, patients visit their community pharmacist 12 times more often than they do their primary care provider
Study finds that health care providers need to reinforce the importance of annual mammograms among patients who survived breast cancer.

Sarah Wheeler, PharmD, BCOP, clinical pharmacy specialist in Hematology/Oncology at UF Health Shands Cancer Hospital, discusses some of the barriers patients with cancer may experience that oncology pharmacists can help them navigate through.
Medication reconciliation and pharmacist counseling are crucial to clinical management of patients with hematologic malignancies.

Recent studies that indicated the efficacy of IVIG in patients with myelin oligodendrocyte glycoprotein antibody-associated disease used pediatric cohorts, with few evaluations of the treatment approach in adults.

Study captures the immune effect of the third dose of the COVID-19 vaccine in patients with plasma cell disorders and blood cancers.
Pharmacists who educate patients with multiple sclerosis and expand their understanding and knowledge of the condition empowers them to take part in a more active role in managing their health.
Melissa Johnson, MD, program director of Lung Cancer Research at the Sarah Cannon Research Institute, discusses her presentation at the Community Oncology Alliance 2022 conference on new drugs in the non-small cell lung cancer space.

Jim Schwartz, RPh, executive director of pharmacy operations for Oncology Pharmacy Services, discusses his presentation at the Community Oncology Alliance 2022 conference on the new world of oral cancer drug dispensing.

Alpelisib is the first FDA-approved treatment for PROS, which is a range of rare conditions characterized by overgrowths and blood vessel anomalies.

Pharmacy workflow changes in the preparation of chemotherapy products can reduce patient chair time.
The value of a patient-first approach and telehealth for addressing the complex health care coordination needs of patients with rare pulmonary diseases has never been more critical than during the COVID-19 pandemic.

The entire pharmacy profession and all those interested in medication safety need to coalesce around the need for systemic change in community pharmacy workplace environments.

Sirolimus is the first topical treatment to gain FDA approval for facial angiofibroma associated with tuberous sclerosis complex.

Axicabtagene ciloleucel (Yescarta) approved for patients with large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or relapses within 12 months of first-line chemoimmunotherapy.

Sarah Wheeler, PharmD, BCOP, clinical pharmacy specialist in Hematology/Oncology at UF Health Shands Cancer Hospital, discusses some of the barriers that patients with cancer may encounter following their diagnosis.

Session at the Hematology/Oncology Pharmacy Association Annual Conference 2022 focuses on methods to improve emotional exhaustion among pharmacists and other health care providers.

Rucaparib (Rubraca) significantly improved progression-free survival when administered as maintenance treatment in newly diagnosed patients with advanced ovarian cancer.

Ted Okon, MBA, executive director of the Community Oncology Alliance (COA), discusses key highlights from the in-person COA 2022 conference.

These include progression-free survival, time to treatment failure, and time to tumor progression, according to a presentation at the Hematology/Oncology Pharmacy Association conference.