
Patients frequently overuse proton pump inhibitors for unapproved indications in place of more expensive and powerful agents.

Patients frequently overuse proton pump inhibitors for unapproved indications in place of more expensive and powerful agents.

John Fanikos, RPh, MBA, director of pharmacy at Brigham and Women's Hospital, talks about what clinical guidelines pharmacists should refer to when selecting a novel oral anticoagulant.

Hy-Vee has awarded 164 One Step Garden Grants totaling $164,000 to community organizations and Hy-Vee stores this spring as part of the Hy-Vee One Step program.

The FDA's open comment period on the return of genetic testing results will be closing March 31, 2016.

Dennis Williams, PharmD, FAPhA, discusses why it's so important to use proper technique for intranasal steroids.

Dennis Williams, PharmD, FAPhA, discusses which clinical situations can be managed by nonpharmacological treatments.

Michael Cawley, BS, PharmD, RRT, CPFT, FCCM, professor of clinical pharmacy at the University of the Sciences, discusses some important patient-related factors to consider when selecting among available inhalers.

Prospective pharmacy students interested in law may want to consider applying to the top schools based on Multistate Pharmacy Jurisprudence Examination (MPJE) passing rates.

Hospira Inc, a Pfizer company, is voluntarily recalling 1 lot of 8.4% sodium bicarbonate injection due to the potential presence of particulate matter.

There may be a few I"s in interprofessional education (IPE), but that doesn't mean it isn't a team sport.

University of California, San Diego, Skaggs School of Pharmacy and Pharmaceutical Sciences (UCSD SSPPS) has a 3-part mission: education, research, and service.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.

There was some degree of unmet demand for pharmacists nationally in January and December, which was a slight improvement from November, when the number of pharmacists looking for work seemed to exceed the number of pharmacist jobs available.
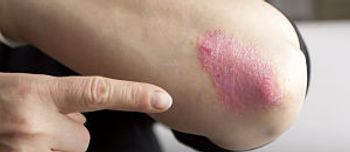
The FDA has approved Eli Lilly and Company's ixekizumab (Taltz) as a treatment for moderate to severe plaque psoriasis.

Children's Miracle Network Hospitals is proud to announce that is has partnered with United Networks of America, one of the largest providers of value-added managed care products and services in the country.

Older adults are increasingly putting themselves at risk for adverse effects by concurrently taking multiple prescription drugs and supplements.

You're not being paid to do everything right. You're being paid to do just one thing right.
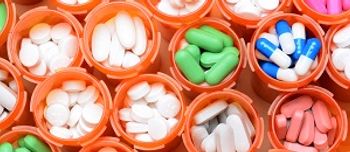
Pharmacists should be aware of the latest drug trend report from the nation's largest pharmacy benefits manager to better inform patients of any shifts in pricing.

Bristol-Myers Squibb recently announced that it's acquiring Padlock Therapeutics, Inc.

A potential addition to technology-based diabetes care is transdermal metformin.

Patients receiving follow-up care more than 6 weeks after a heart attack are much less likely to stick to their medication regimen.
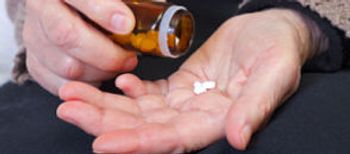
A pharmacy error turned fatal after a staff member allegedly dispensed Florence Frost's medication to an 86-year-old patient named Margaret Forrest.

Mother-to-child HIV transmission drives pediatric AIDS incidence, and risk of transmission increases as the mother's viral load increases.

Failure is a hard thing to admit.

A multifaceted collaboration of health care professionals, including pharmacists, prescribers, and patients, are working together to reduce the impact of this crisis.

State regulation of telepharmacy varies depending on the market-driven needs within each particular state.
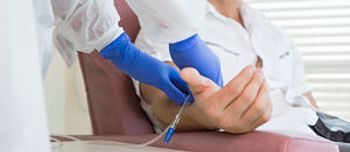
The FDA is proposing a ban on powdered gloves in most medical situations.

A pair of North Dakota State University (NDSU) pharmacy students won first prize and the People's Choice Award at the school's 2016 Innovation Challenge.

John Fanikos, RPh, MBA, director of pharmacy at Brigham and Women's Hospital, discusses new trends pharmacists should know about atrial fibrillation management.

Michael Cawley, BS, PharmD, RRT, CPFT, FCCM, professor of clinical pharmacy at the University of the Sciences, demonstrates proper technique for inhaler devices.