
Nonstop standing is a common complaint among pharmacists, and it has been shown to cause muscle fatigue.

Nonstop standing is a common complaint among pharmacists, and it has been shown to cause muscle fatigue.

As Arizona public health officials work to stop the spread of a measles outbreak, CVS Health is offering the measles-mumps-rubella vaccine to people living in Maricopa and Pinal Counties, the areas exposed to confirmed cases of the illness.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.

To ensure patient access to newer, more affordable versions of biologic medicines, the Food and Drug Administration should avoid requiring a statement of biosimilarity on the product label, a group of leading pharmaceutical supply chain organizations stated in a letter submitted to the agency today

Pharmacogenomic testing may provide an option for improving pharmacotherapy in patients with mental illness.

Pharmacy school tuition is expensive.

Due to manufacturing delays, Pfizer is shipping only 30% of its normal monthly supply of penicillin G benzathine injectable suspension (Bicillin L-A) until July 2016.

Since the introduction of the Cockcroft-Gault equation to calculate creatinine clearance, there has been debate about which weight to use in the equation.

The CDC keeps tabs on the most common causes of death for educational and research purposes.

Pharmacy residencies can be very demanding, but they can also help you stand out in this competitive pharmacy job market.

The FDA has issued new guidance for the use of the first-line diabetes drug metformin in patients with renal impairment.
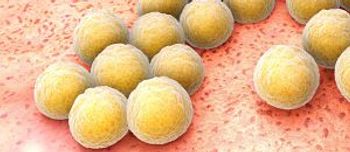
Pediatric patients may now use ceftaroline fosamil (Teflaro) as a treatment for acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia.

The FDA issued a warning today alerting providers and patients about the potential risk of serious burns and scarring associated with the use of a migraine patch.

Nominations are now being accepted for the American Pharmacists Association annual awards and honors program.

For the first time in 20 years, prescriptions for opioids are steadily declining.

Here are the top trending pharmacy stories you may have missed in May:

The FDA has approved linagliptin and metformin hydrochloride extended-release (Jentadueto XR) tablets for adults with type 2 diabetes.

The Ohio Northern University Raabe College of Pharmacy chapter of the National Community Pharmacists Association received the Dennis Ludwig Memorial Scholarship in Government Affairs for having the highest student attendance at the annual Congressional Pharmacy Summit.

Are you an HIV expert or amateur?

The American College of Cardiology, American Heart Association, and Heart Failure Society of America recently updated guidelines for pharmacological heart failure treatment, focusing specifically on 2 new agents.

Meijer has opened a new 192,000-square-foot supercenter in Sturgis, Michigan, bringing Southern Michigan shoppers better access to a full-service pharmacy that makes family health care a priority.

This is the second part of a 2-part series on how to retire after 25 years of working as a pharmacist.

A pharmacy technician is pleading not guilty to larceny of drugs from her Massachusetts pharmacy.

The FDA has approved fluciclovine F 18 (Axumin), which is a radioactive diagnostic agent for injection that can be used to detect recurring prostate cancer.

Learn the business of pharmacy as The Pharmacy Podcast Show releases a new show format and digital health content marketing services.

With the release of the 300th episode of the Pharmacy Podcast Show, we offer several new #DigitalHealth content marketing support services for our pharmaceutical industry sponsors, announce the new Pharmacy Podcast Network, and interview Emilie Ray, President with McKesson Pharmacy Technology and Services division in Pittsburgh, Pennsylvania.

After some deliberation, a student with cerebral palsy will be able to attend North Dakota State University School of Pharmacy if she can meet certain requirements.

Because diabetes can affect many parts of the body, there's a lot to remember for self-management.

This article highlights several key therapeutics areas for PCSK9 inhibitors that every pharmacist should know.

Rite Aid Corporation has announced the grand opening of its first new distribution center in 16 years.